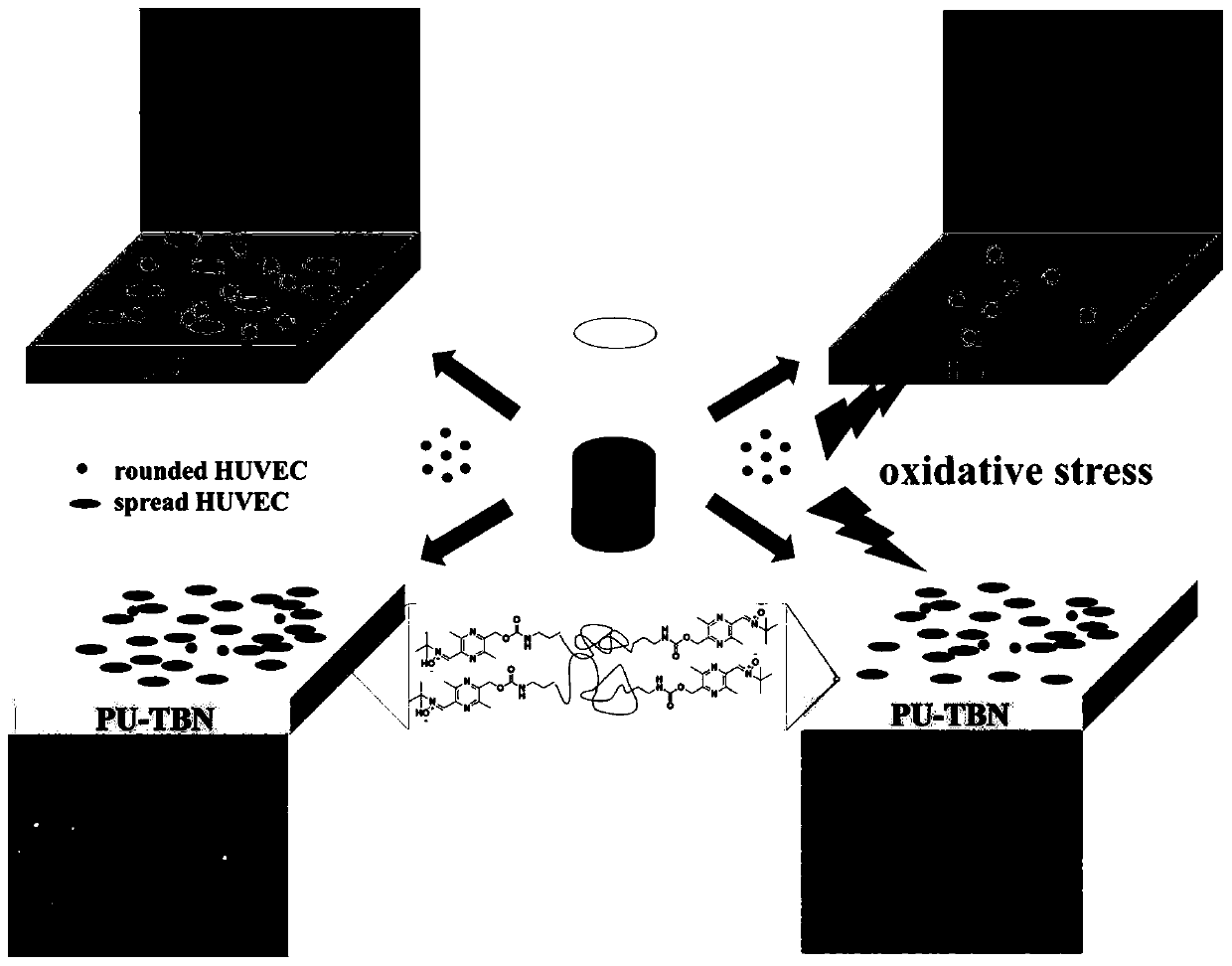
Difunctional polyurethane and preparation method and application thereof
A polyurethane and bifunctional technology, applied in the field of bifunctional polyurethane and its preparation, can solve the problem of inability to eliminate oxidative stress at the implantation site of blood vessels, and achieve the effects of good processability and relieving oxidative stress.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
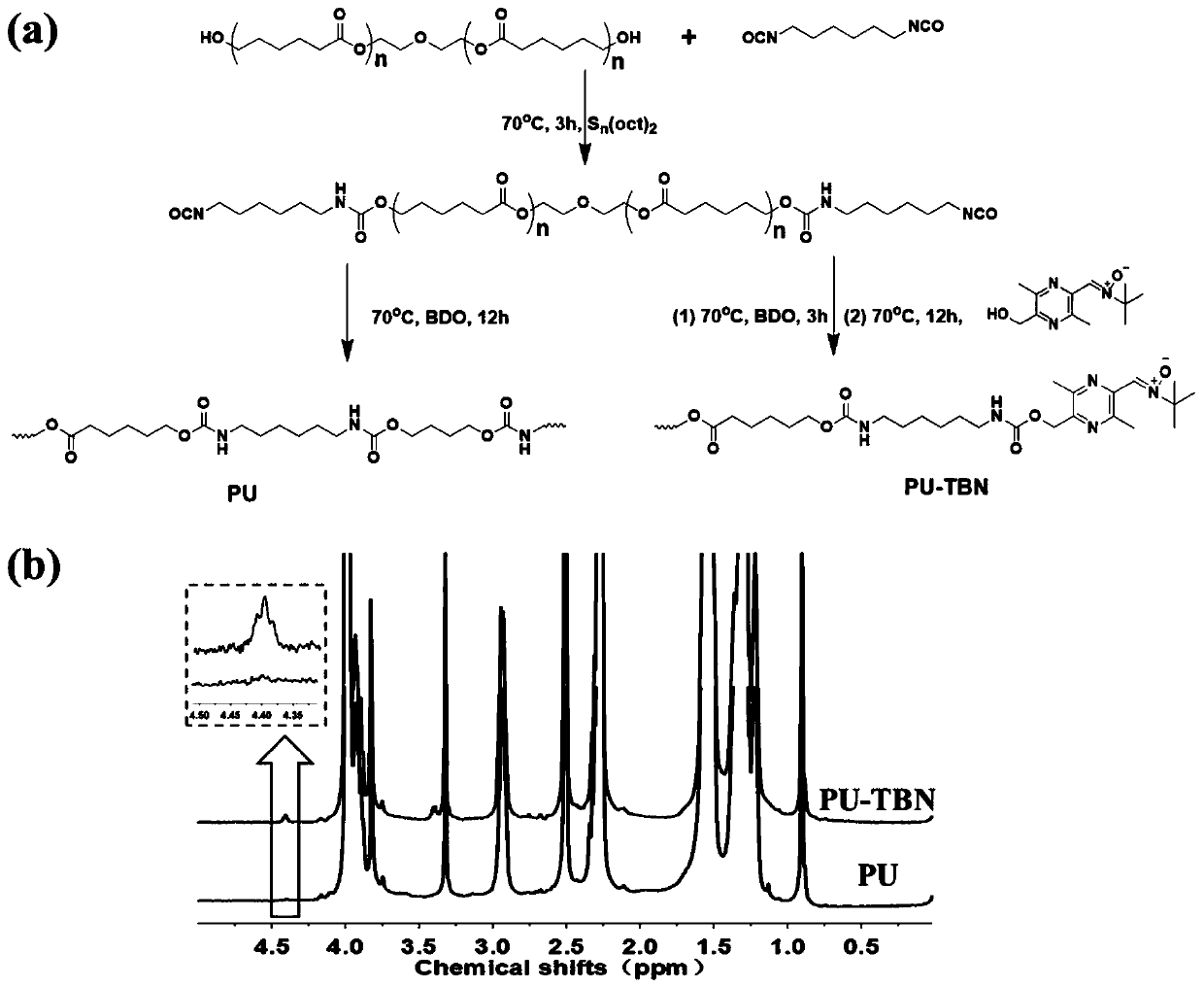
[0052] The preparation method of bifunctional polyurethane of the present invention comprises the following steps:
[0053] Step 1. Add polyol and dry solvent to the reactor, dehydrate, add isocyanate under the protection of inert atmosphere, and react at 60-80°C for 2-3h to obtain polyurethane prepolymer;
[0054] Step 2, adding small molecular polyols to the polyurethane prepolymer, reacting at 60-80°C for 3-6 hours, to obtain polyurethane with NCO groups at the end of the molecular chain;
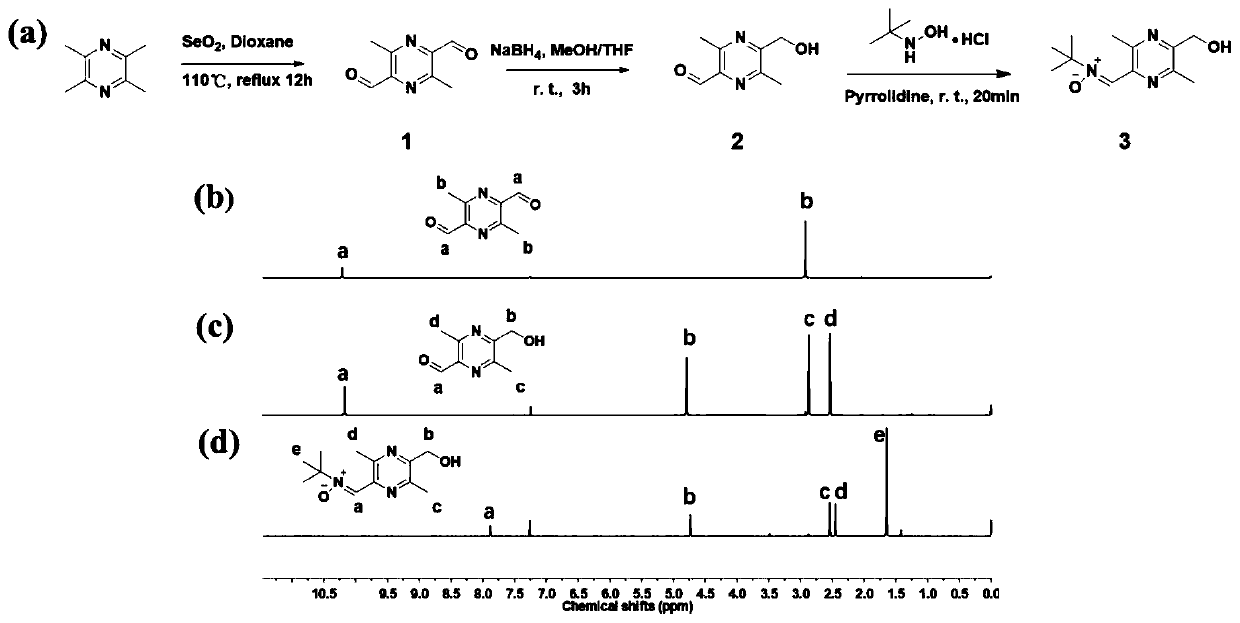
[0055] Step 3: Add ligustrazine-nitrone capping agent to the polyurethane with NCO group at the end of the molecular chain, react at 60-80°C for 6-12h, precipitate in ether, wash with ether for several times, collect the white precipitate, and dry it in vacuum until Constant weight, obtains bifunctional polyurethane;
[0056] The structural formula of the ligustrazine-nitrone blocking agent is shown in formula III:
[0057]
[0058] In the above technical solutions, there are no spe...
Embodiment 1
[0074] Such as figure 2 Shown in (a), the synthesis of bifunctional polyurethane:
[0075] Step 1. Dissolve polycaprolactone polyol (10g, molecular weight 2000 Daltons) in 200mL dry DMF, heat to 70°C, and add 2.52g hexamethylene diisocyanate dropwise under the protection of inert gas and 0.02g of stannous octoate was stirred at 70°C for 3h to obtain the first intermediate.
[0076] Step 2: Add 0.86 g of 1,4-butanediol in 10 mL of dry DMF dropwise to the first intermediate, and stir at 70° C. for 3 h after the addition is complete.
[0077] Step 3: Continue to add 10 mL of TBN-OH (0.47 g) DMF solution dropwise, and continue to react at 70°C for 12 hours, then precipitate the reaction solution in 1 L of ether, rinse with ether for 2-3 times, collect the white precipitate, and dry it in vacuum until Constant weight, to obtain bifunctional polyurethane, denoted as PU-TBN.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com