Compound with phosphodiesterase 4D and acid sphingomyelinase inhibitory activity
A technology of compounds and mixtures, applied in the direction of organic active ingredients, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve problems such as aggravating depression symptoms, slow onset of action, and affecting the treatment effect of depression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of above-mentioned compound is:
[0039]
[0040] Intermediate 1 reacts with thionyl chloride to prepare acid chloride, and then reacts with intermediate 2 to obtain intermediate 3 through acylation reaction, and intermediate 3 reacts with hydroxylamine potassium prepared from hydroxylamine hydrochloride to obtain the target compound through aminolysis.
[0041] Wherein, the molar ratio of the intermediate 1 added in step a to thionyl chloride is 1:1 to 1:3, the reaction solvent is one of methylene chloride, tetrahydrofuran or a mixed solvent thereof, and the reaction temperature is from room temperature to the solvent used reflux temperature; the solvent used in the acylation reaction is dichloromethane, tetrahydrofuran, ethyl acetate, pyridine, and the acid-binding agent used is triethylamine, diisopropylethylamine, pyridine, sodium hydroxide, potassium carbonate, carbonic acid One of cesium and sodium bicarbonate.
[0042] The reaction solv...
Embodiment 1
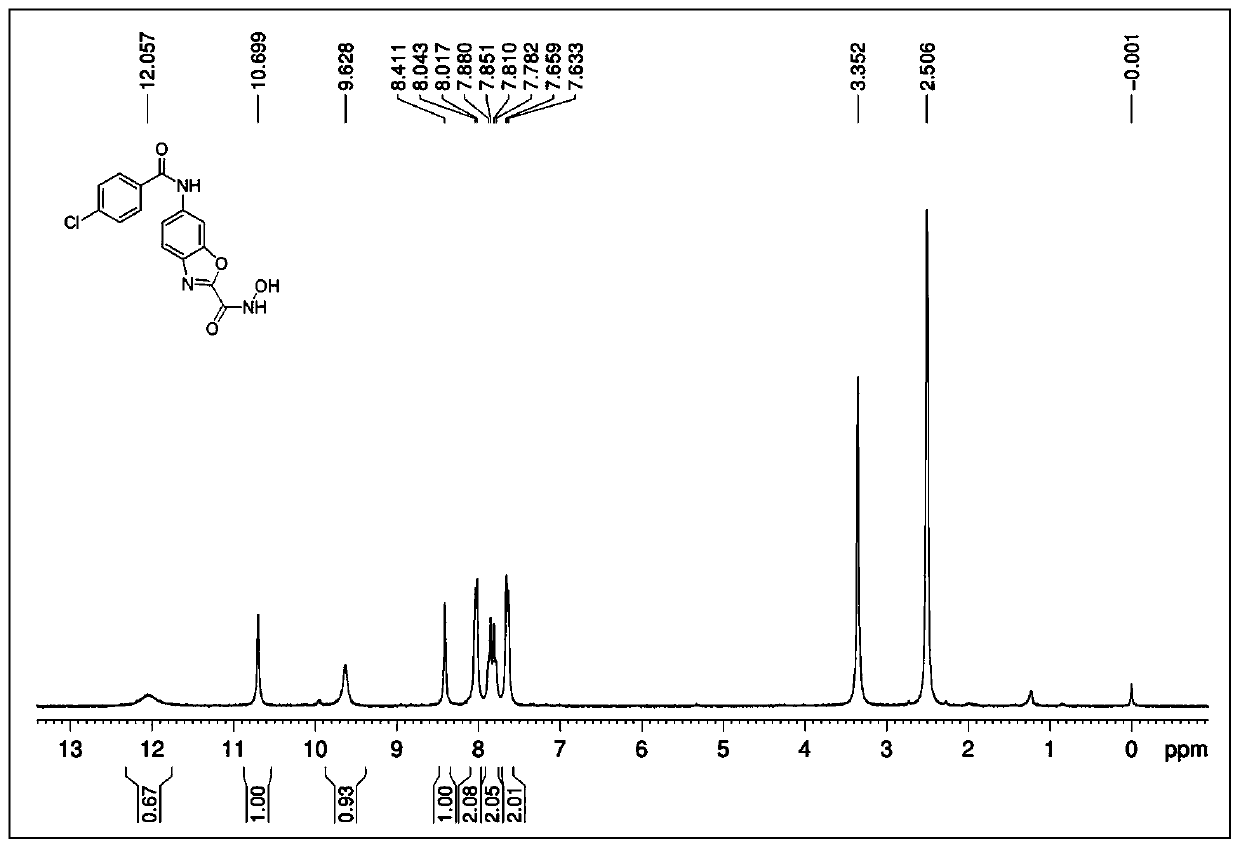
[0076] N-Hydroxy-6-(4-chlorobenzamido)benzo[d]oxazole-2-carboxamide (Ⅱ-1)
[0077] Step 1, Ethyl 6-nitrobenzo[d]oxazole-2-carboxylate
[0078]
[0079] Take 1.60 g (10.38 mmol) of 2-amino-5-nitrophenol into a 100 mL eggplant-shaped flask, and add 30 mL of pyridine dried with potassium hydroxide to dissolve. Under stirring, 7.75 mL (72.67 mmol) of monoethyl oxalyl chloride was added dropwise with a constant pressure dropping funnel. After the dropwise addition was completed, the reaction was heated at 100°C for 4h. The reaction solution was cooled and stirred in an ice-water bath, diluted with 20 mL of ethyl acetate, and added dropwise with 15% hydrochloric acid. Add 100 mL of saturated sodium chloride solution for dilution, extract three times with ethyl acetate, combine the organic phases, and dry the organic phases with anhydrous sodium sulfate. The organic phase was made into sand, and silica gel column chromatography (petroleum ether: ethyl acetate = 8:1) gave 1.23 g...
Embodiment 2
[0091] N-Hydroxy-6-(3,4-dimethoxybenzamido)benzo[d]oxazole-2-carboxamide (Ⅱ-2)
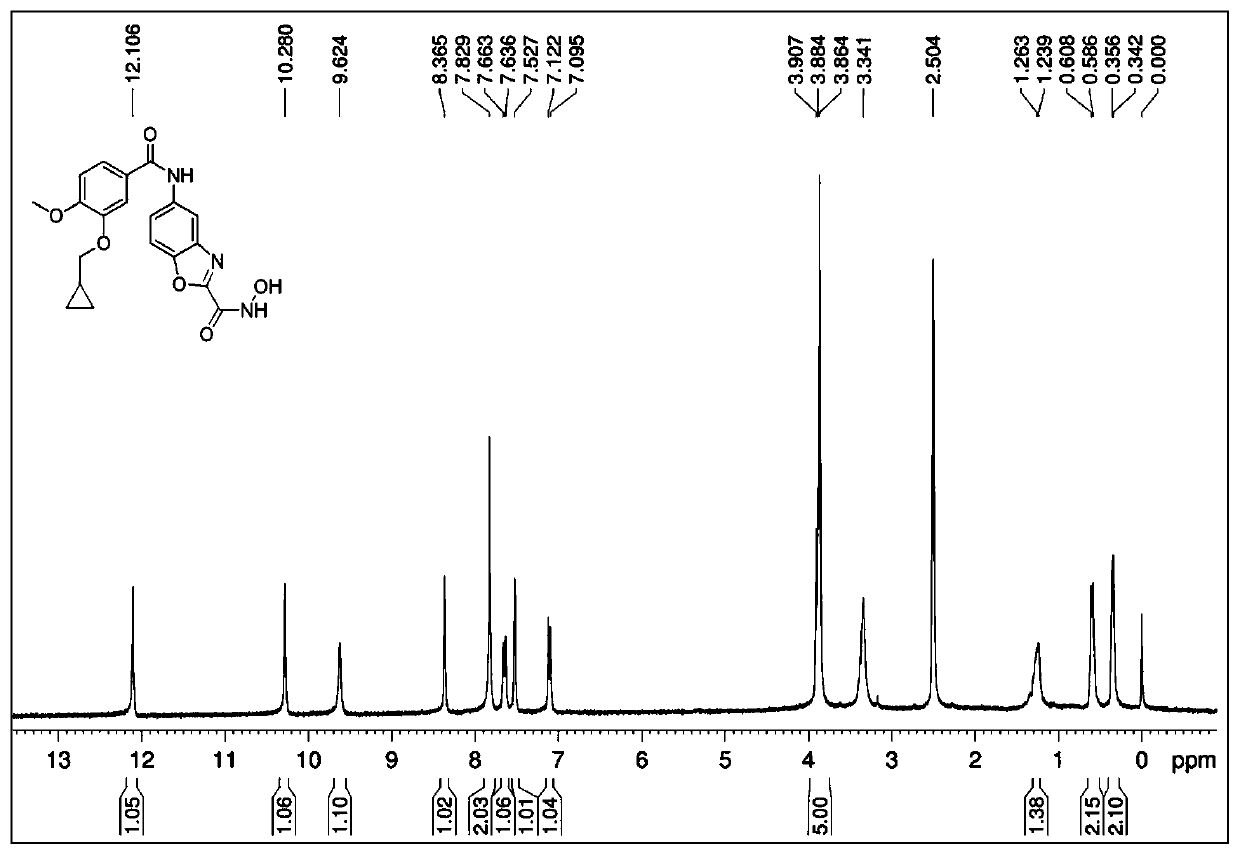
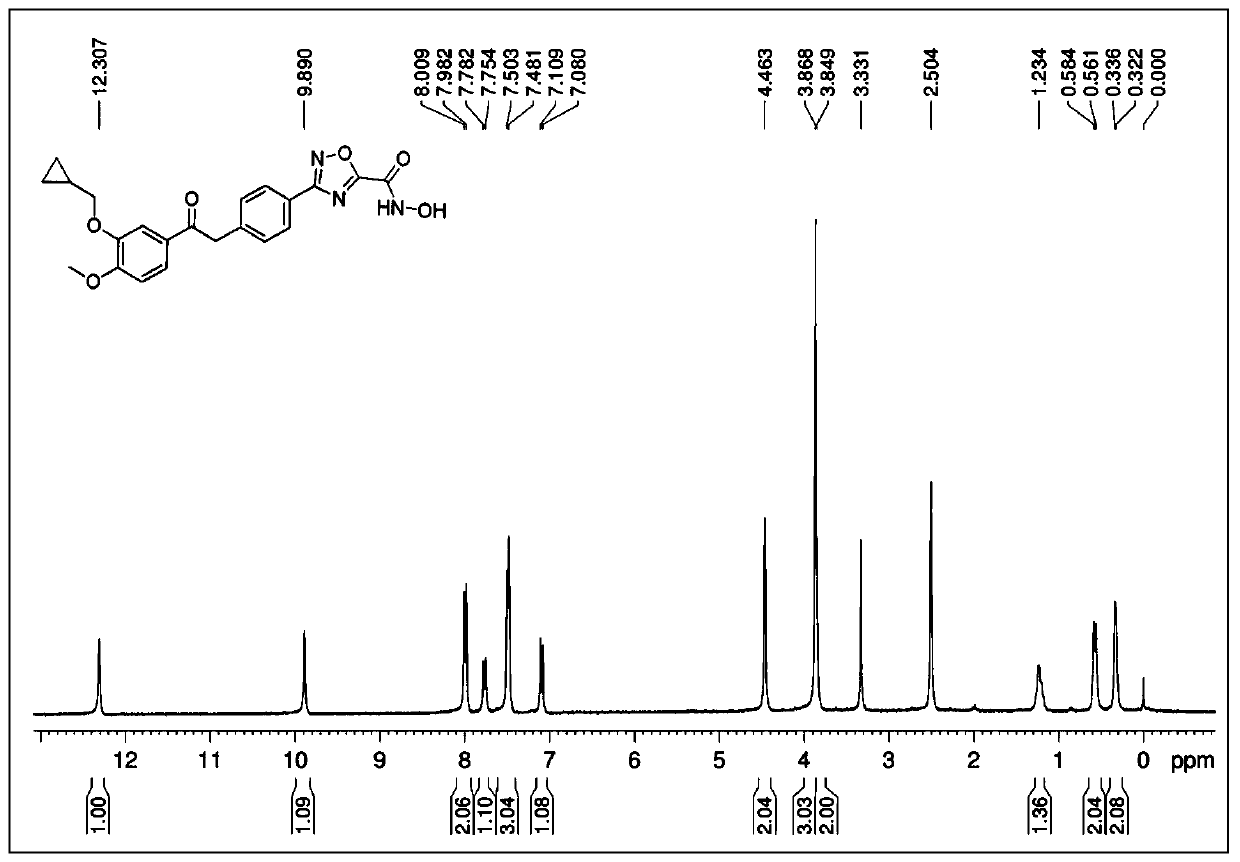
[0092] Referring to the synthesis method of II-1 in Example 1, 4-chlorobenzoic acid in step 3 was replaced by 3,4-dimethoxybenzoic acid to obtain 50 mg of white solid with a yield of 40.8%. M.P.198-201℃, 1 H-NMR (300MHz, DMSO-d 6 ): δ12.03(s,1H),10.42(s,1H),9.59(s,1H),8.41(s,1H),7.85(d,J=8.7Hz,1H),7.78(d,J= 8.7Hz, 1H), 7.67(d, J=8.4Hz, 1H), 7.57(s, 1H), 7.12(d, J=8.7Hz, 1H), 3.86(s, 6H); HRMS(ESI-TOF) :calcd for C 17 h 15 N 3 o 6 [M+H] + 358.1039, found 358.1036.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com