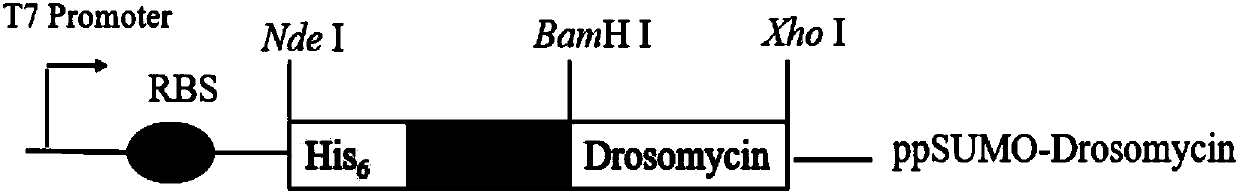
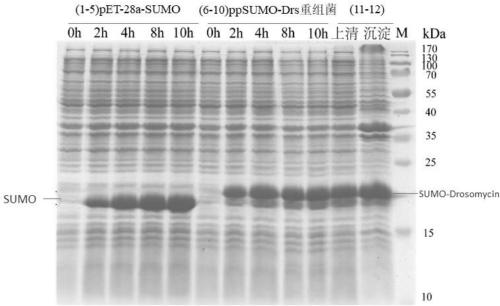
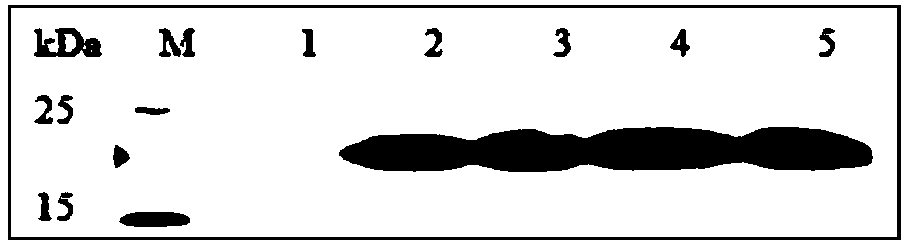
Antifungal peptide Drosomycin, preparation method and application thereof
A technology of antifungal peptides and receptor bacteria, applied in antifungal agents, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problems of low yield of insect antimicrobial peptides, cumbersome extraction steps, and low natural content, etc. Achieve the effects of easy industrial production, high market value, and high expression output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Primer design and SUMO-Drosomycin sequence acquisition
[0046] 1. The acquisition of Drosomycin gene SEQ.ID NO.1
[0047]The gene sequence corresponding to the mature Drosomycin peptide is 132bp, as shown in SEQ.ID NO.1, encoding 44 amino acids, the predicted isoelectric point of the protein is 5.45, and the size is 4.9kDa. Drosomycin has a complex structure, with 8 Cys forming 4 pairs of disulfide bonds, which is not suitable for expression in prokaryotic systems. Therefore, according to the above-mentioned characteristics of the peptide, the characteristics of the gene and the expression system, the codons of the Drosomycin gene are first optimized, and the optimized Drosomycin nucleotide sequence is shown in SEQ.ID NO.5. According to the optimized nucleotide sequence, three mutually complementary long primers drs-1, drs-2 and drs-3 were designed respectively, and Drosomycin was directly synthesized by PCR.
[0048] Firstly, the codon-optimized Drosomycin gene w...
Embodiment 2
[0119] For the antimicrobial peptide Drosomycin (Drs) obtained in Example 1, utilize the conventional agar plate hole diffusion method (plate inhibition zone method) to treat Neurospora crassa (N.crassa) and Fusarium oxysporum (F.oxysporum ) was used as the test bacteria to test the antibacterial activity.
[0120] The plate antibacterial test results are as follows: Figure 7 As shown, PBS and a single SUMO protein have no inhibitory effect on the two tested bacteria, while the isolated antimicrobial peptide Drosomycin has an inhibitory effect on the two bacteria when it is above 3 μg, and it shows obvious antibacterial activity at 15 μg .
[0121] Drosomycin and control (PBS) at a concentration of 5 μg / μL were used to act on Neurospora crassa and Fusarium oxysporum respectively. After 10 hours of treatment, the hyphae were fixed with paraformaldehyde, and the JSM-6360LV scanning electron microscope was used to examine the Observed and photographed at 1200 and 3700 times, t...
Embodiment 3
[0123] The CCK-8 method was used to detect the toxicity of the in vitro recombinant protein Drosomycin described in Example 1 to mammalian cell 293T and mouse embryonic fibroblast 3T3-L1, and it was found that it did not affect the proliferation of 293T and 3T3-L1 cells, indicating that the antifungal peptide Drosomycin has no toxic effect on mammalian cells. The result is as Figure 9 As shown, it can be seen that mammalian cells 293-T and 3T3-L1 were treated with 0, 10 μg / mL, 40 μg / mL, and 160 μg / mL of Drosomycin, and 24 hours later, 293-T and 3T3-L1 cells were detected by CCK8 kit As for the proliferation ability, it can be seen from the figure that even if the concentration of Drosomycin is increased to 160 μg / mL, the proliferation ability of 293-T and 3T3-L1 cells is maintained at about 100%. It can be seen that Drosomycin has no toxic effect on mammalian cells 293-T and 3T3-L1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Isoelectric point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com