Medical polyurethane material containing lactone group, preparation method thereof, and medical catheter
A technology of polyurethane materials and medical catheters, applied in medical science, surgery, etc., can solve the problems of reduced inner diameter of catheters, reduced input flow rate of medicinal liquid, lack of thermoplastic processability, etc., to achieve compression load reduction, increase hydrophilicity, Effect of Rigidity Reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention provides a kind of preparation method of medical polyurethane material described in above-mentioned technical scheme, comprises the following steps:
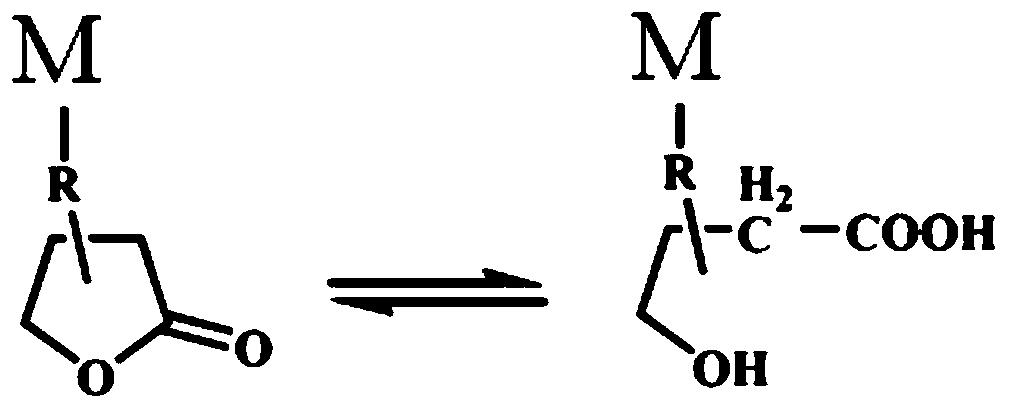
[0033] Mix 4,4`-diphenylmethane diisocyanate, butanediol, polytetrahydrofuran, substances containing butyrolactone groups and lubricants, and then extrude and granulate to obtain medical polyurethane containing butyrolactone groups Material;
[0034] In the present invention, the lubricant is selected from ethylene bisstearamide.
[0035] In the present invention, a twin-screw extruder with an aspect ratio of 44 / 1 is preferably used for extrusion granulation. In the present invention, the extrusion granulation temperature is preferably 200-220°C, more preferably 205-215°C; in a specific embodiment, the extrusion granulation temperature is 210°C.
[0036] The present invention provides a medical catheter, which is produced by extruding the medical polyurethane material described in the above technical...
Embodiment 1
[0042] Slowly add 0.5 mol (51.0 g) of 3-hydroxybutyrolactone to 0.5 mol (125.1 g) of 4,4'-diphenylmethane diisocyanate (MDI) at 80°C, and react for 2 hours. 0.5 mol (45.5 g) of 3-amino-1,2-propanediol (APD) was slowly added dropwise thereto, and the reaction was continued for 2 h to obtain 0.5 mol (221.6 g) of lactone polyol. In a reaction kettle at 100°C, 10mol (2502.4g) of MDI was reacted with 2mol (2000g) of polytetrahydrofuran (PTMO) with a molecular weight of 1000g / mol for 20min, and then 7.5mol (675.5g) was added thereto under vigorous stirring. The mixed solution of butanediol and the lactone polyol of 0.5mol (221.6g), and 16.2g ethylene bisstearamide, pass through the twin-screw extruder that length-diameter ratio is 44 / 1 after fast mixing uniformly at 210 Extrusion granulation at ℃. A medical polyurethane material with a lactone content of 9.2 mmol / g and a hard segment content of 57.5 wt % was obtained. Press the polyurethane material into a sheet with a thickness o...
Embodiment 2
[0045] Slowly add 0.7 mol (71.4 g) of 3-hydroxybutyrolactone to 0.7 mol (175.1 g) of 4,4'-diphenylmethane diisocyanate (MDI) at 80°C, and react for 2 hours. 0.7 mol (63.7 g) of 3-amino-1,2-propanediol (APD) was slowly added dropwise thereto, and the reaction was continued for 2 h to obtain 1.0 mol (310.2 g) of lactone polyol. In a reaction kettle at 100°C, 10mol (2502.4g) of MDI was reacted with 2.5mol (2500g) of polytetrahydrofuran (PTMO) with a molecular weight of 1000g / mol for 20 minutes, and then 6.8mol (612.8g) was added to it under vigorous stirring. ) of butanediol and 0.7mol (310.2g) of the lactone polyol mixed solution, and 16.2g ethylene bisstearamide, after rapid mixing, the length-to-diameter ratio is 44 / 1 through the twin-screw extruder Extruded and granulated at 210° C., a medical polyurethane material with a lactone content of 11.8 mmol / g and a hard segment content of 52.4 wt % was obtained. Press the polyurethane material into a sheet with a thickness of 0.45m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com