Kluyvera intermedia tyrosine phenol-lyase mutant and application thereof
A technology of Kluyverella and tyrosine phenol, which is applied in the field of biocatalysis, can solve the problem of low TPL enzyme activity, and achieve the effect of simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Cloning of embodiment 1 tyrosine phenol lyase gene (KI-TPL)
[0024] By designing primers YW118 (SEQ ID NO.5: 5'CACTGGATCCATGATTTACCC GGCCGAACC 3') and YW119 (SEQ ID NO.6: 5'TTCGAAGCTTTTAAATGT ATTCAAAGCGTGCGG 3'), Kluyvera intermedia (Kluyvera intermedia, purchased from China Industrial The genome of Microorganism Culture Collection Management Center) was used as a template to amplify KI-TPL, and the cloned sequence is shown in SEQ ID NO.1. The amplification system was 50 μL (2×PrimeSTAR Max DNA polymerase (purchased from Beijing Baoriyi Biological Co., Ltd.) 25 μL, 1 μL YW118+1 μL YW119, 1 μL K.intermedia genome, distilled water to 22 μL), pre-denaturation at 95°C for 3 minutes, Denature at 98°C for 10 seconds, anneal at 58°C for 15 seconds, extend at 72°C for 2 minutes (30 cycles), and extend again at 72°C for 10 minutes.
[0025] After recovering the KI-TPL fragment obtained above using a gel recovery kit (purchased from Beijing Baoriyi Biological Co., Ltd.), it was...
Embodiment 2
[0026] The construction of embodiment 2 mutation library
[0027] Using an adjustable error-prone PCR kit (purchased from Beijing Tianenze Gene Technology Co., Ltd.), with primers YW117 and YW118, PCR random mutation of KI-TPL was carried out, and 2-4 mutation sites were controlled. The mutation system was 30 μL (3 μL 10 × Error-prone Mix, 3 μL error-prone dNTP, 3 μL MnCl 2 , 1 μL K.intermedia genome, 1 μL Taq DNA polymerase, 1 μL YW118+1 μL YW119, 17 μL distilled water). PCR amplification conditions were pre-denaturation at 94°C for 3 minutes, denaturation at 94°C for 1 minute, annealing at 58°C for 1 minute (30 cycles), and extension at 72°C for 1 minute. The KI-TPL fragment amplified by the above random mutation was digested with restriction endonucleases BamH I and Hind III, ligated with the pET-28a(+) vector cut with the same BamH I and Hind III, and transferred into BL21 , to construct a mutant library.
Embodiment 3
[0028] Example 3 Screening of mutant library
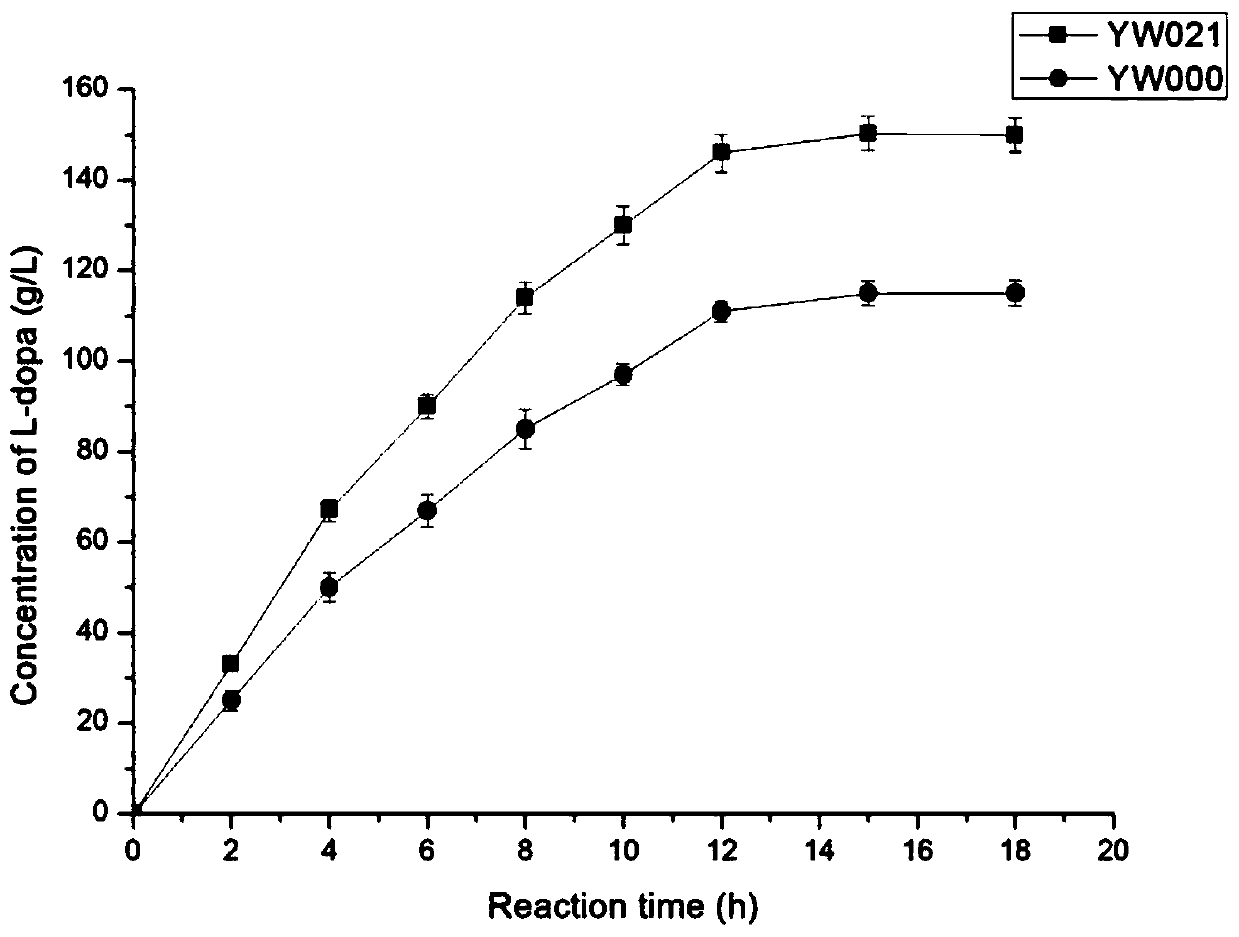
[0029]The screening of the mutant library was carried out as described in (XL Tang, etal. Analytical Biochemistry. 2018.560:7–11), using the principle of color reaction between pyruvate and salicylaldehyde. The specific steps are as follows: the mutants were picked and inoculated in a test tube containing 3 mL of LB medium, cultured for 24 hours, then added with 0.2 mM IPTG for induction for 12 hours, and centrifuged at 4000 rpm for 5 minutes to collect the bacteria. Add the bacteria to 600μL reaction solution (10g / L pyruvate, 12.5g / L catechol, 20g / L ammonium acetate, 1g / L EDTA, 0.12g / L PLP, 2g / L sodium sulfite) and react for 20 minutes After the reaction was completed, 400 μL of 1M HCl was added to terminate the reaction. Subsequently, 100 μL reaction solution was mixed with 200 μL 5M NaOH solution, 100 μL 5% (V / V) salicylaldehyde, 600 μL ddH 2 O to mix well, then measure the absorbance value at 465nm. According to the magnitu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com