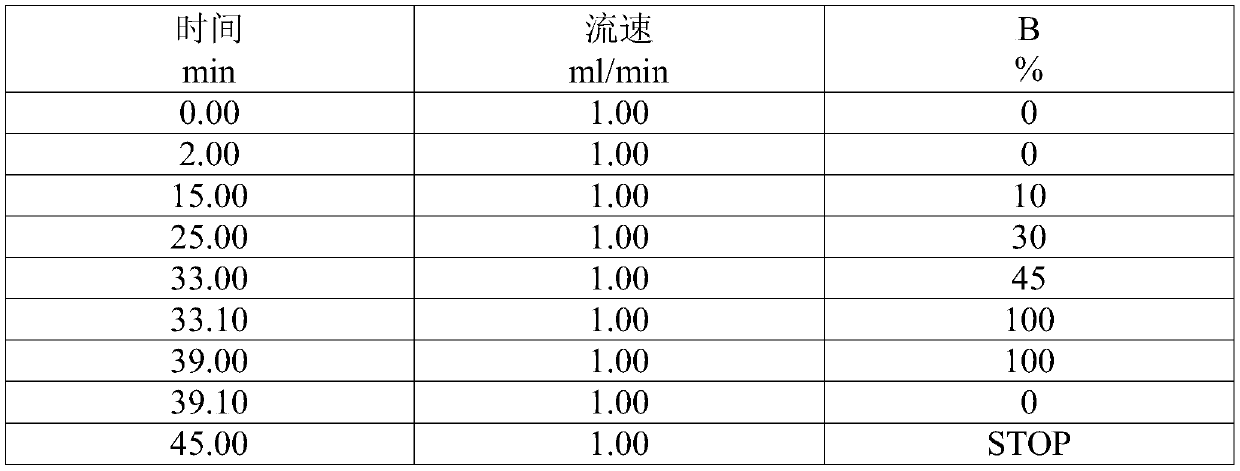
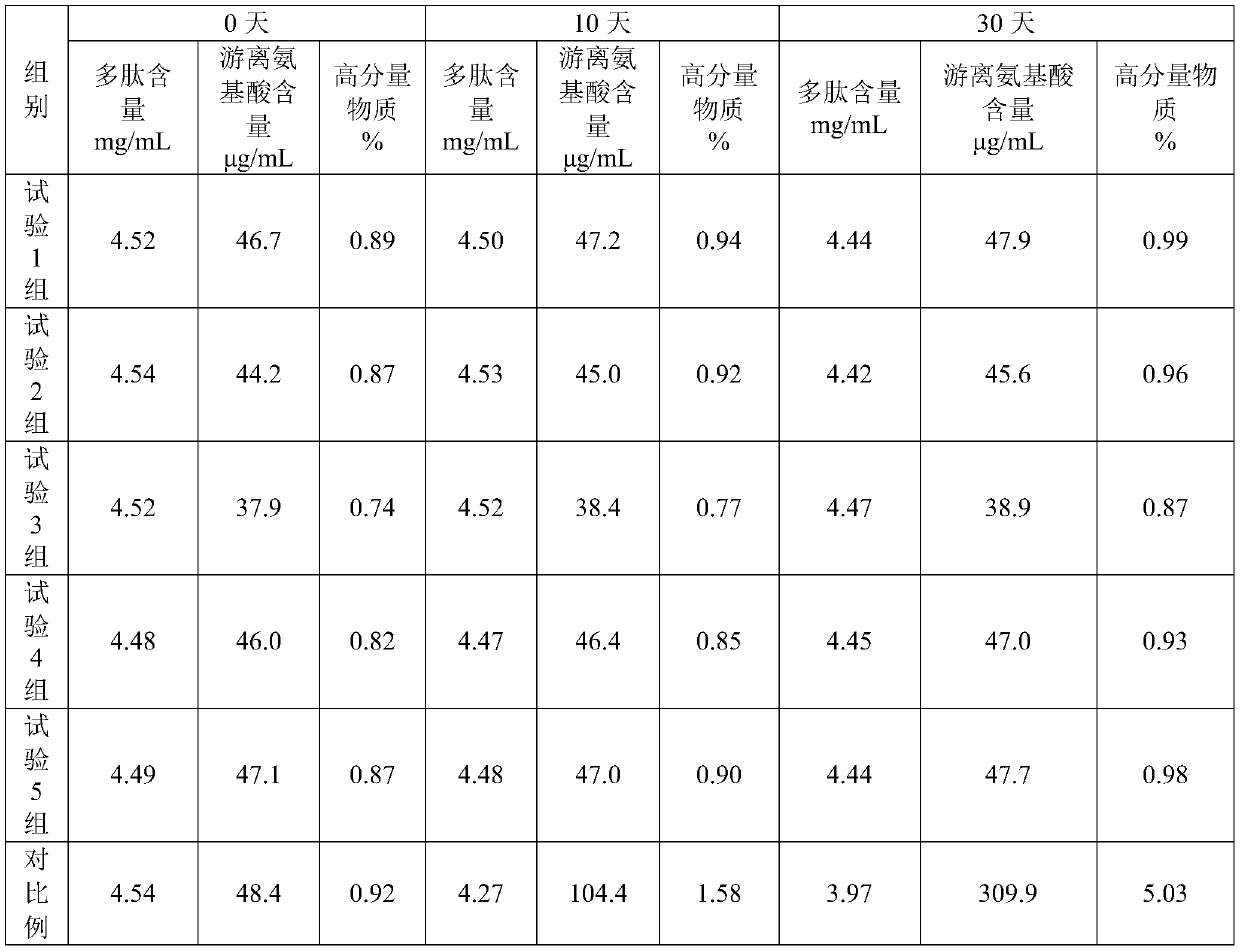
Preparation method of cervus and cucumis polypeptide injection preparation
A technology for injection preparations and deer melon polypeptides, applied in the field of deer melon polypeptide injection preparations, can solve the problems of reduced active ingredient content, increased clinical allergic reactions, and reduced bioavailability, and achieves low product cost, excellent quality, and reduced medication costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take the deer bone, remove the bone marrow, crush, weigh, add 1.5% deer bone weight compound protease and 2 times deer bone weight water for injection, compound protease is pepsin and papain with a weight ratio of 1.85:1; extract at 40°C for 3 hours, Filtrate, adjust the pH of the filtrate to 4.5 with citric acid, let stand, filter, adjust the pH to 8.5 with sodium citrate, add 0.1 times the weight of deer bone pancreatin, hydrolyze at 40°C for 3 hours, filter, and citrate the filtrate The pH value is adjusted by acid to 6.5, followed by an ultrafiltration system with a molecular weight cut-off of 100,000, a molecular weight cut-off ultrafiltration system with a molecular weight of 10,000, and a molecular weight cut-off ultrafiltration unit with a molecular weight of 8,000 to perform ultrafiltration, retain the permeate, and freeze-dry to obtain peptides extracted from deer bone .
[0025] The preparation method of the polypeptide extracted from melon seeds is:
[0026] Tak...
Embodiment 2
[0029] The preparation method of the polypeptide extracted from deer bone is: take deer bone, remove bone marrow, crush, weigh, add 2.5% deer bone weight compound protease and 4 times deer bone weight water for injection, compound protease is a pepsin and a weight ratio of 2.35:1 Papain: Extract for 1 hour at 50℃, filter, adjust the pH of the filtrate to 6.0 with citric acid, let stand, filter, adjust the pH to 9.0 with sodium citrate for the filtrate, add 0.3 times the weight of deer bone pancreatin, and hydrolyze at 50℃ Filter for 1 hour, adjust the filtrate to pH 7.5 with citric acid, and use the ultrafiltration system with a molecular weight cut-off of 100,000, an ultrafiltration system with a molecular weight of 10,000, and an ultrafiltration unit with a molecular weight of 8,000 to perform ultrafiltration and retain the permeate. , Freeze-drying, get the polypeptide extracted from deer bone.
[0030] The preparation method of the polypeptide extracted from melon seeds is: t...
Embodiment 3
[0033] The preparation method of polypeptide extracted from deer bone is: take deer bone, remove bone marrow, crush, weigh, add 2.0% deer bone weight compound protease and 3 times deer bone weight water for injection, compound protease is pepsin and weight ratio 2.05:1 Papain; extract for 1 to 3 hours at 40℃~50℃, filter, adjust the pH of the filtrate to 5.5 with citric acid, let stand, filter, adjust the pH of the filtrate to 8.8 with sodium citrate, add 0.2 times the weight of deer bone pancreas Enzyme, hydrolyze at 45°C for 2 hours, filter, adjust the filtrate to pH 7.0 with citric acid, and use the ultrafiltration system with a molecular weight cut-off of 100,000, a molecular weight cut-off ultrafiltration system with a molecular weight of 10,000, and a molecular weight cut-off ultrafiltration unit to perform ultrafiltration. , Retain the permeate, freeze-dry, and get the polypeptide extracted from deer bone.
[0034] The preparation method of the polypeptide extracted from me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com