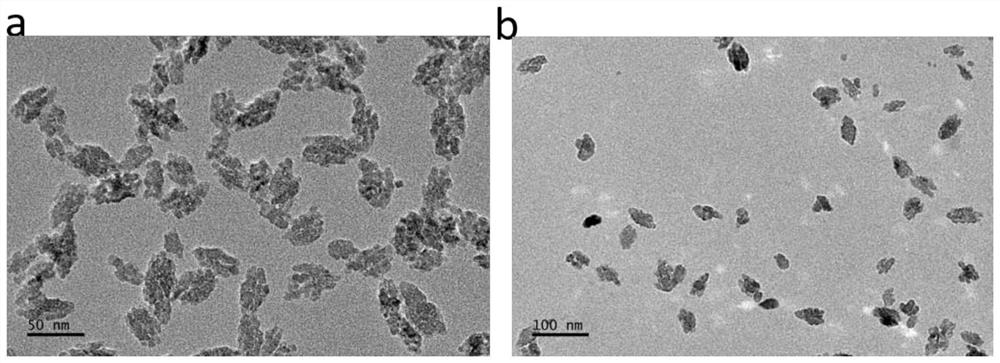
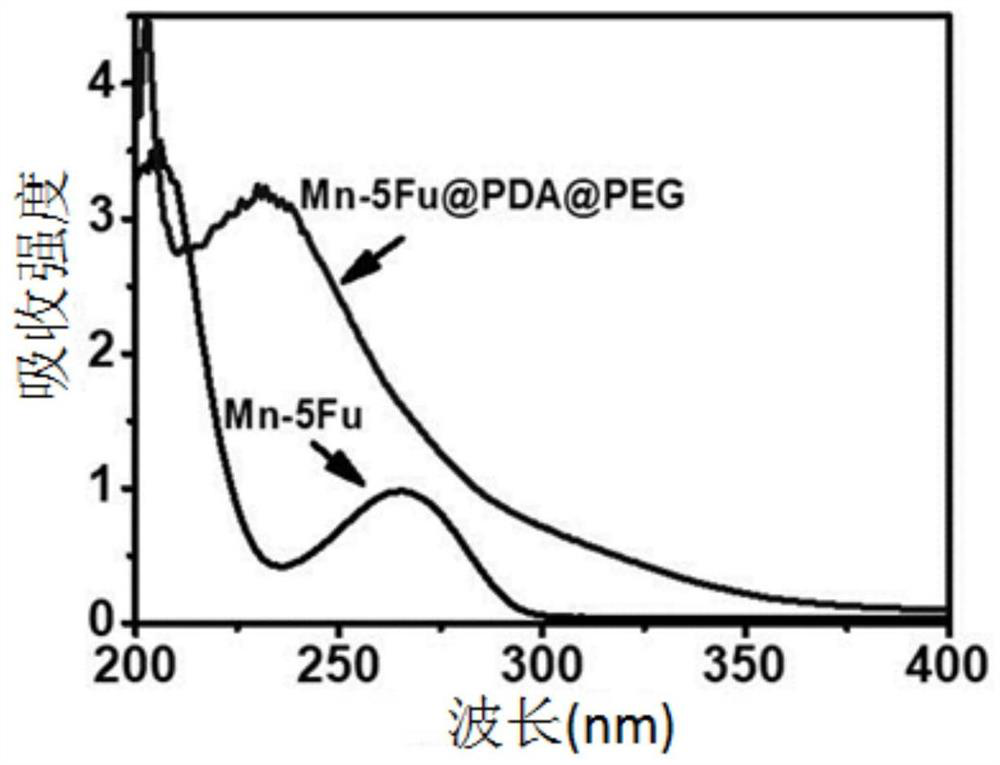
A kind of preparation method and application of pentafluorouracil nano drug preparation
A technology of pentafluorouracil and nano-drug, applied in the directions of drug combination, pharmaceutical formulation, anti-tumor drug, etc., can solve the problems of poor tumor cell selectivity, unfavorable cell endocytosis, strong toxic and side effects, etc., to improve bioavailability and effective Dosage, the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Synthesis of Mn-pentafluorouracil nanoparticles (Mn-5Fu nanoparticles)
[0023] 0.2g (1mmol) of MnCl 2 4H 2 O and 0.13g (1mmol) of 5-Fu were placed in 25mL of DMF solvent, and the solution was ultrasonicated until the solution was clear; then, 0.25mL triethylamine was added to the above solution, and ultrasonication was continued for 1h; after the ultrasonication was completed, the entire solution was placed at 100°C Heat in an oven for 1 hour, then continue ultrasonication for 20 minutes, then filter, wash the filter cake three times with DMF and water respectively, finally obtain Mn-5Fu, disperse it in water, and store it at room temperature.
[0024] 2. Preparation of pentafluorouracil nanomedicine preparations (Mn-5Fu@PDA@PEG)
[0025] Take 8 mL of Mn-5Fu dispersed in Tris buffer (the concentration of Mn-5Fu is 10 mM, the pH of Tris buffer is 8.5), add 0.2 mL of freshly prepared dopamine aqueous solution (2 mg / mL), and stir at room temperature for 1 h, the solu...
Embodiment 2
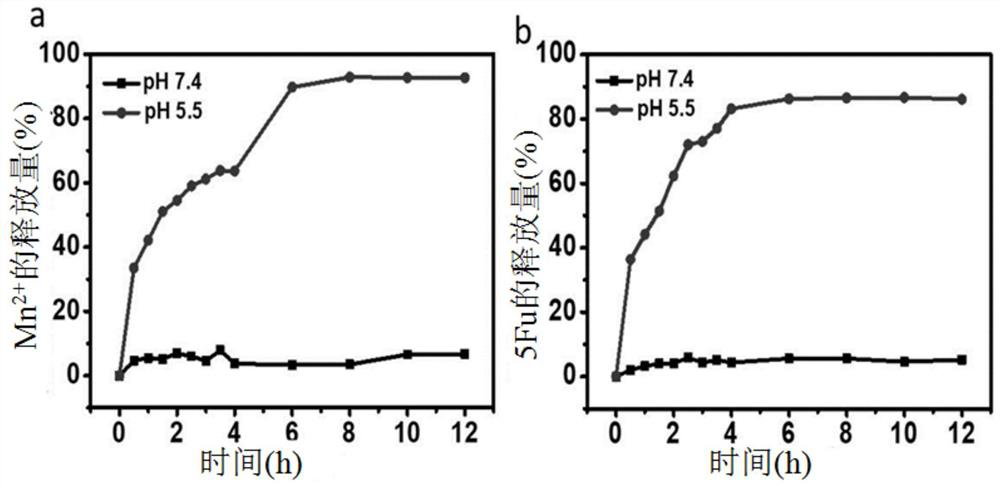
[0028] Embodiment 2Mn-5Fu drug release
[0029]Disperse the 10mM (according to 5Fu calculation) aqueous solution of Mn-5Fu nanoparticles into the PBS buffer solution of pH 5.5or 7.4, after a period of time (0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6 , 8, 10 and 12h) Take out 0.2mL of the solution and centrifuge to leave the supernatant. The supernatant was used to measure the concentration of pentafluorouracil quantitatively by ultraviolet absorption spectrometry, and to measure the concentration of manganese ions by inductively coupled plasma mass spectrometry (ICP-MS). Calculate the amount of drug and manganese ion released at different times. The result is as image 3 As shown, in the environment of pH 7.4, the amount of manganese ions or drugs released by Mn-5Fu does not exceed 10%; while in the environment of pH 5.5, the release of manganese ions and drugs is about 86% and 81%; it shows that Mn-5Fu Manganese ions and drugs can be released under acidic conditions, indicating ...
Embodiment 3
[0030] Example 3 Cytotoxicity Test
[0031] Mouse breast cancer cells (4T1) were inoculated into a 96-well plate, cultured in a cell incubator for 24 hours, and then washed twice with DPBS. Cells were incubated with different concentrations of Mn-5Fu (concentrations were calculated based on 5Fu, respectively 1, 10, 50, 100, and 200uM) for 24h or 48h, discard the cell culture medium, add fresh medium and MTS staining After incubating for 30 min in a cell culture incubator, measure the UV absorbance of each well of cells at 490 nm with a microplate reader. Calculate cell viability. Take the 5Fu solution according to the above concentration as a control.
[0032] The result is as Figure 4 As shown, the survival rate of cells incubated with 5Fu was higher than that of cells treated with Mn-5Fu, especially at concentrations of 50, 100, and 200uM, the survival rate of cells incubated with 5Fu was higher than that of cells treated with Mn-5Fu double the cell viability. This sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com