A method for preparing single-layer porous cobalt oxyhydroxide nanosheets by electrochemical oxidation
A technology of cobalt oxyhydroxide and electrocatalytic oxidation, applied in the field of electrochemistry, can solve problems such as unreported cobalt oxyhydroxide, and achieve the effects of high porosity, low energy consumption and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1.8 g of tetrahydrate cobalt is dissolved in 100 mL of ethylene glycol, 0.6 g of polyvinylpyridone, 5 mL distilled water, thoroughly stirred 30 min to all dissolved to form a transparent clear solution. The transparent solution was transferred to a 250 mL of crystallization tank, sealed, and reacted at 220 ° C for 6 h. After the reaction is completed, the hydrotherm is rinsed quickly to the room temperature to room temperature. The resulting suspension was centrifuged to give precipitation, and then the resulting precipitate was washed 3 times with anhydrous ethanol, dried at 50 ° C for 10 h, i.e., a pink precursor.
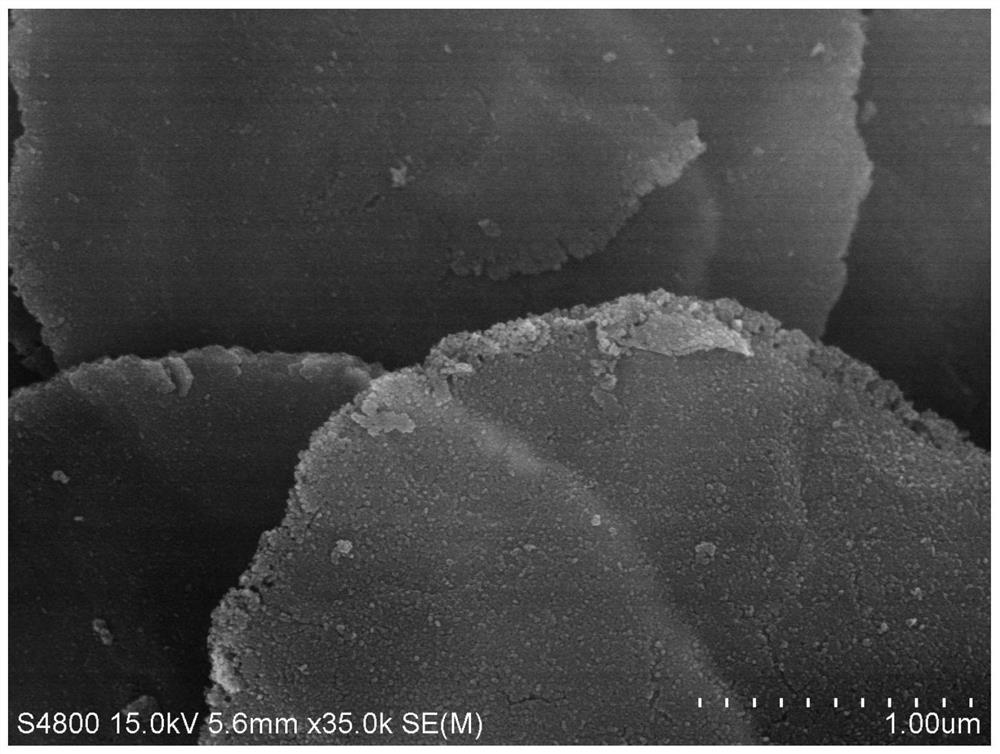
[0027] The above-mentioned pink precursor 100 mg was taken to 1 * 1 cm carbon paper as the working electrode, and the 0.1 M KOH solution is an electrolyte. In constant current density (10mA cm -2 Electrolytic 3 h can be obtained from a single layer of porous hydroxy cobalt oxide. The SEM map of the product is attached figure 1 . Depend on figure 1 It can be se...
Embodiment 2
[0029] 0.9 g of cobalt nitrate was dissolved in 100 mL of ethylene glycol, and 0.3 g of polyvinylpyrone is added, and stir well to all dissolved. 5 ml of distilled water was added, stirred for 30 min to form a transparent solution. The transparent solution was transferred to a 250 ml of crystallization tank, sealed, and reacted at 200 ° C for 12 h. After the reaction is completed, the hydrotherm is rinsed quickly to the room temperature to room temperature. The resulting suspension was centrifuged to give precipitation, and the resulting precipitate was washed 3 times with isopropyl alcohol, and the pink precursor was obtained at 50 ° C for 10 h.
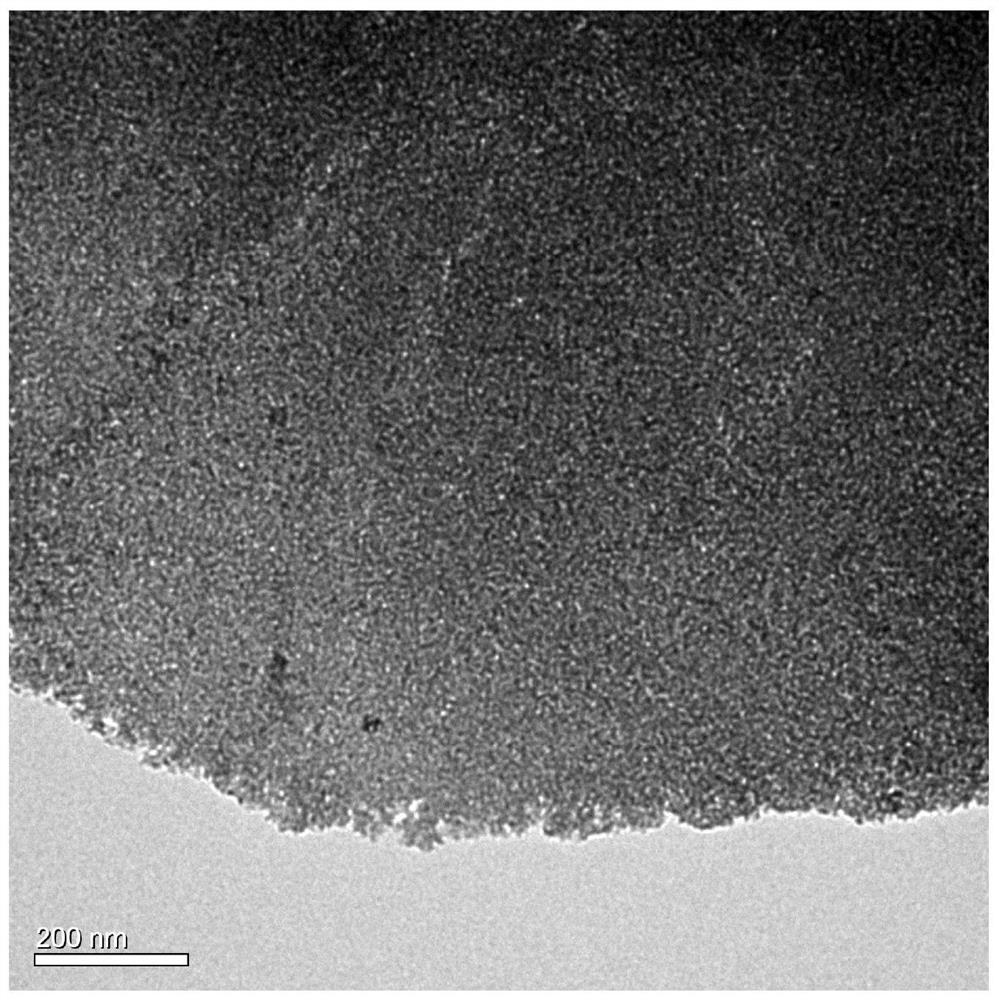
[0030] The pink precursor 120 mg was taken, dispersed onto a carbon paper of 2 * 2 cm, as the working electrode, and 1 M KOH solution as an electrolyte. In constant current density (5mA cm -2 Electrolytic 6 h can be obtained from a single layer porous hydroxy cobalt oxide, and its TEM is attached. figure 2 . Depend on figure 2 It can be...
Embodiment 3
[0032] 0.5 g of cobalt chloride was dissolved in 50 ml of ethylene glycol, and 0.3 g of polyvinylpyrol alkone was added, and stirred well until all dissolved. 5 ml of distilled water was added, stirred for 30 min to form a transparent solution. The transparent solution was transferred to a 250 ml crystallization tank, sealed, at 150 ° C for 24 h. After the reaction is completed, the hydrotherm is rinsed quickly to the room temperature to room temperature. The resulting suspension was centrifuged to obtain precipitation, and the resulting precipitate was washed 3 times with tert-butanol, dried at 50 ° C for 10 h, i.e., a pink precursor.
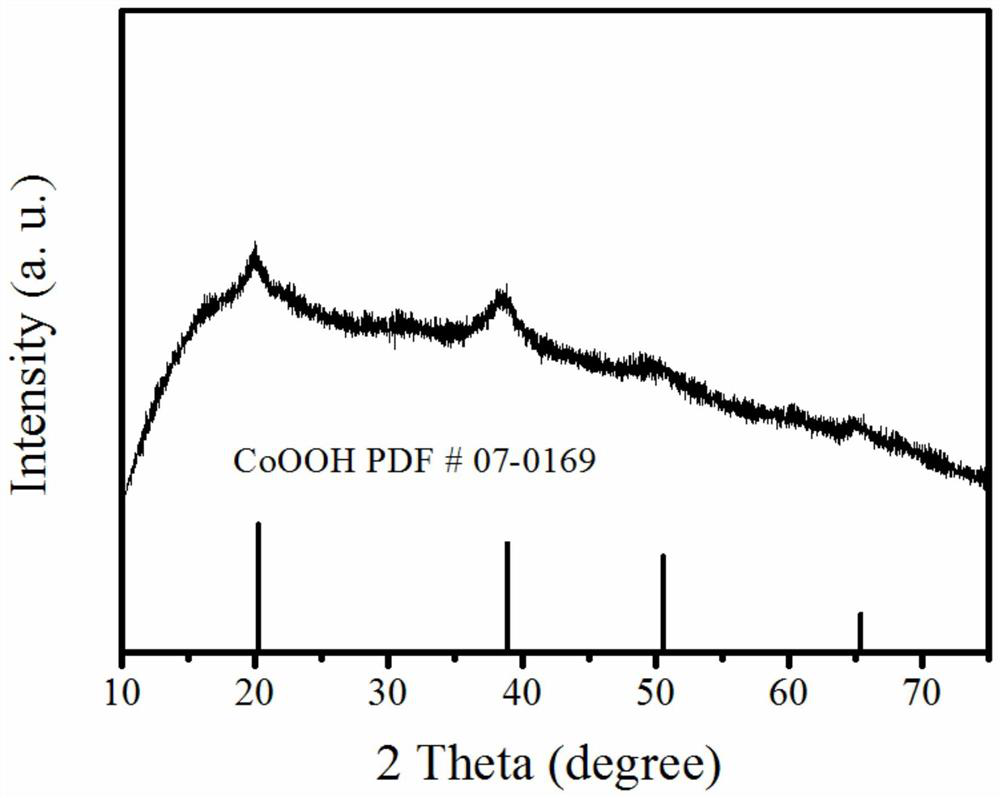
[0033] Take the above-described pink precursor 200 mg, dispersed onto a carbon paper of 3 * 3 cm, as the working electrode, and 5M KOH solution as an electrolyte. In constant current density (20 mA cm -2 The electrolytic 1 h can be obtained from a single layer of porous hydroxy cobalt oxide, and the XRD figure is attached. image 3 . Depend on imag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com