Redox catalysts for the oxidative cracking of hydrocarbons, methods of making, and methods of use thereof
A catalyst, catalyst metal technology, applied in carbon compound catalysts, metal/metal oxide/metal hydroxide catalysts, hydrocarbons and other directions, can solve the problems of unstable performance of catalysts, no reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0097] Embodiment 1: Li promotes La x Sr 2-x FeO 4-δ Core-shell oxygen-supported catalysts for the oxidative dehydrogenation of ethane under a cyclic redox scheme
[0098] The chemical cyclic oxidative dehydrogenation of ethane (CL-ODH) utilizes transition metal oxide-based oxygen carriers, i.e., oxygen carrier catalysts, to convert ethane to ethylene under an autothermal cyclic redox scheme. This embodiment provides Li-promoted La for CL-ODH reactions x Sr 2-x FeO 4-δ (LSF) oxygen carrier catalyst. While LSF without Li promoter exhibited low ethylene selectivity, Li addition resulted in high selectivity / yield and good regeneration ability. Up to 61% ethane conversion and 90% ethylene selectivity were achieved with the Li-promoted LSF. Further characterization revealed that the Li-promoted LSF oxygen-supported catalyst was composed of LiFeO 2 (disordered rock salt) and LSF (Ruddlesden-Popper) phase composition. In addition, the surface of the oxygen-supported catalyst...
Embodiment approach 2
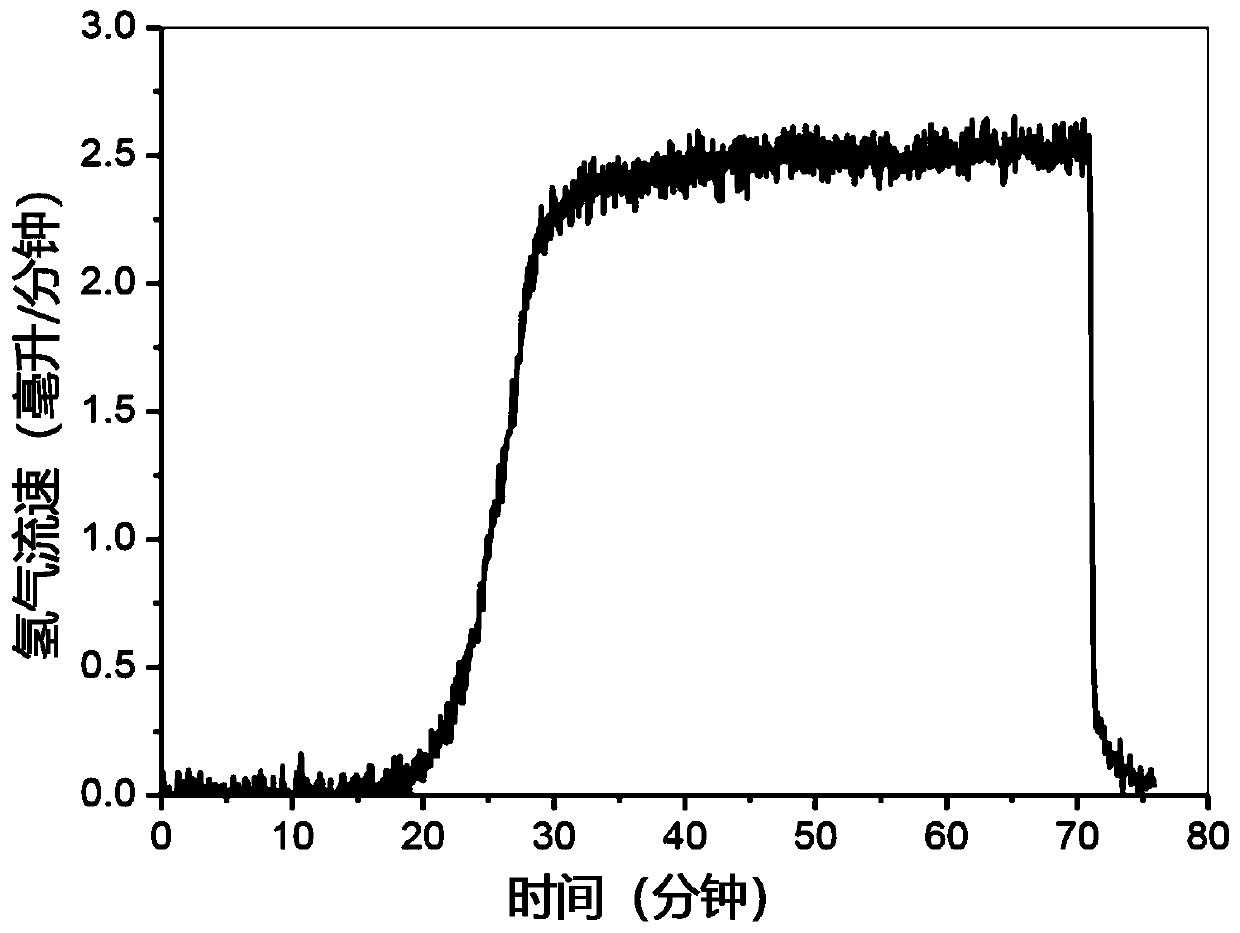
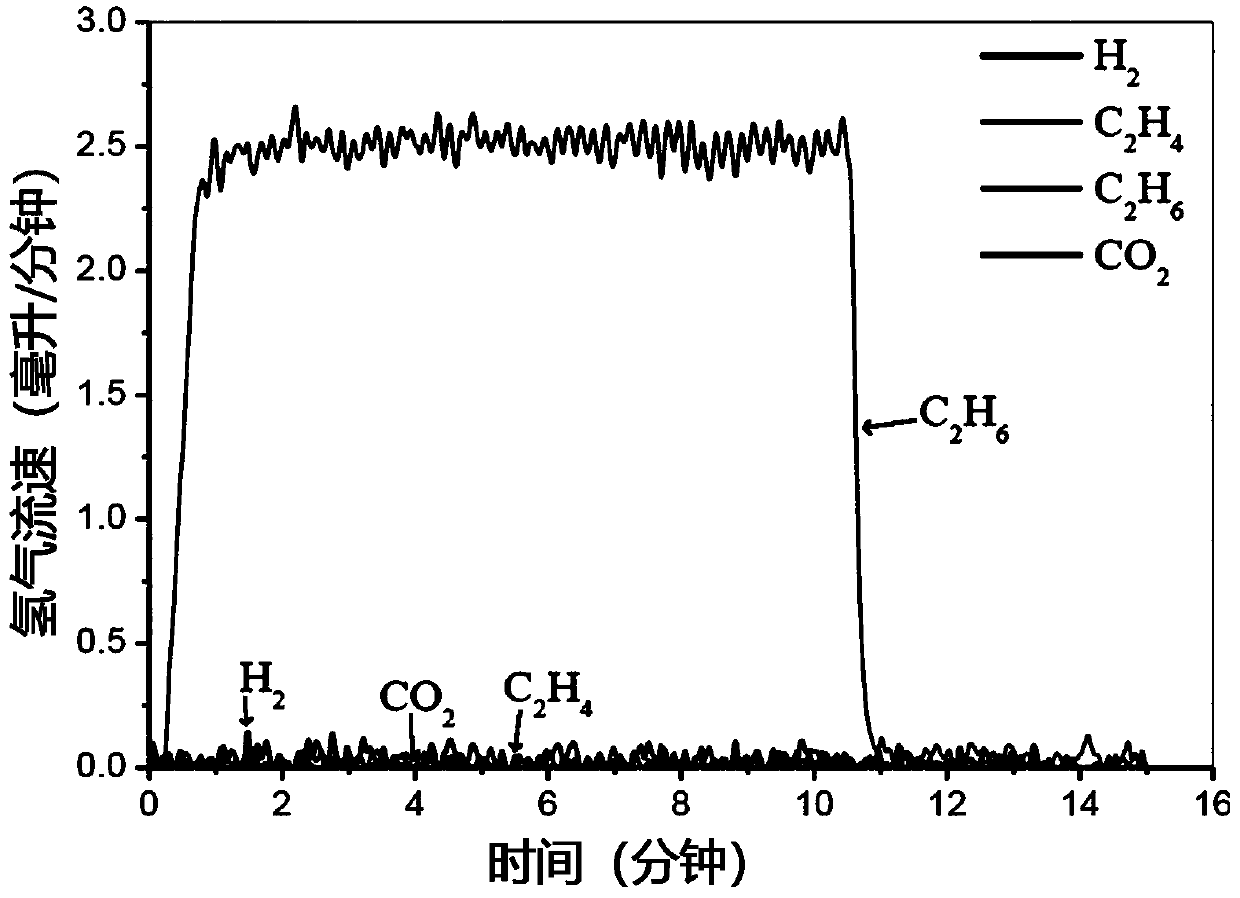
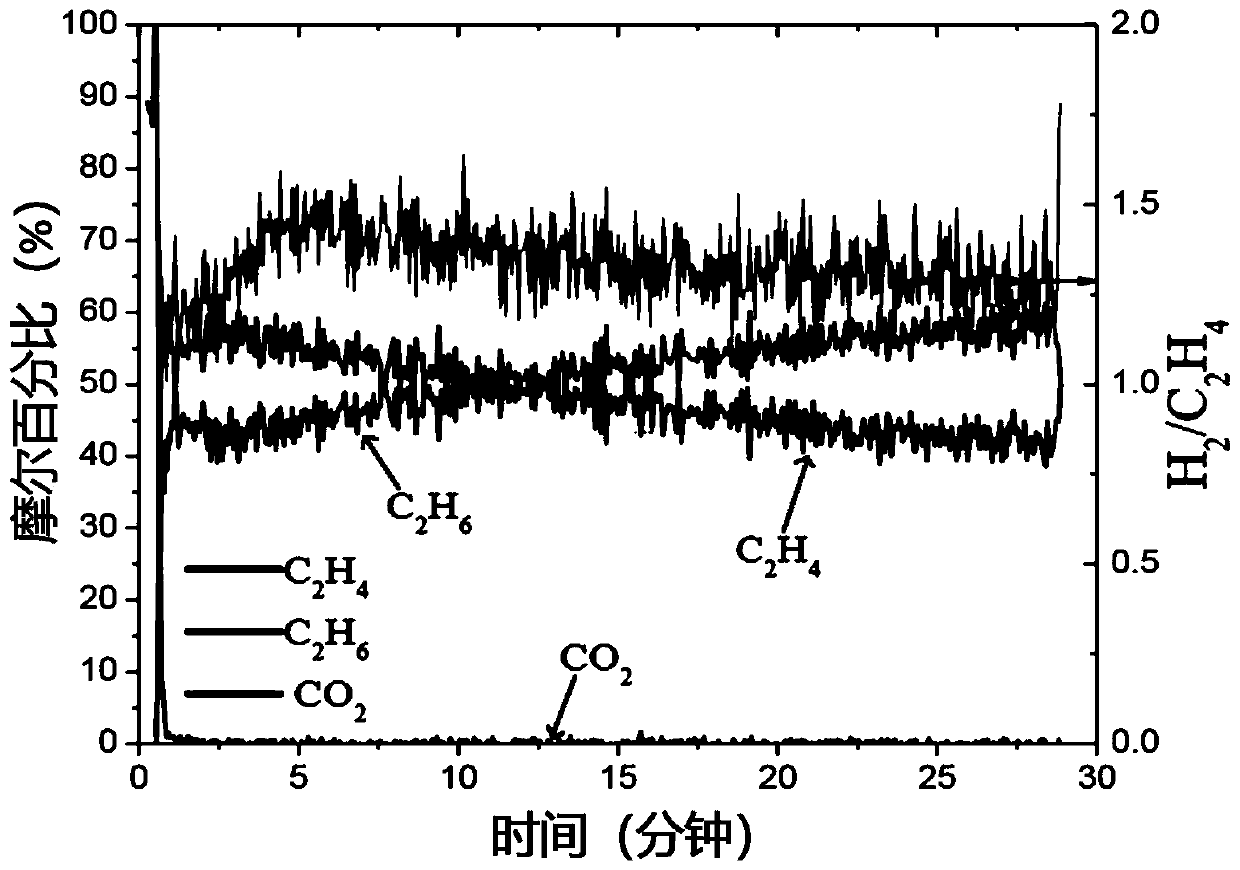
[0141] Preparation of La by a modified Pechini method 0.6 Sr 1.4 FeO 4 (LSF), in which stoichiometric La-, Sr- and Fe-nitrate precursors were dissolved in water and heated with citric acid to form viscous gels. The resulting mixture was dried and calcined in air, followed by LiFeO 2 :LSF impregnated at a molar ratio of 2.5:1. Subsequent calcination yields coated LiFeO 2 / LiOH and / or Li 2 LSF of the O mixture. Therefore, the catalyst self-assembles into a core-shell structure in which the oxygen carrier LSF phase is coated with LiFeO 2 / LiOH / Li 2 O coating, which promotes dehydrogenation and hydrogen oxidation while preventing contact with deeply oxidizing species in the core. In the experiment, 0.5g catalyst was loaded into a 1 / 8"ID quartz U-shaped tube reactor, and inert sand was placed on both sides of the bed to control the gas volume in the heating zone. The U-shaped tube reactor was placed in a tube furnace Heat and pulse 37.5 μL ethane (diluted in 63.5 μL argon)...
Embodiment approach 3
[0145] Preparation of Mg6MnO8 doped with sodium and phosphorus; magnesium oxide powder was impregnated in a stoichiometric solution of manganese(II) nitrate and sodium pyrophosphate (equivalent to 1.7 wt% Na), dried at 80 °C and heated at 950 °C Carry out calcification. The prepared catalyst was further doped with Pr to make it constitute a catalyst with a loading rate of 5wt%. 10% n-hexane was equilibrated with argon at 775°C, 0.5 g catalyst was flowed at 150 SCCM for 20 seconds, regenerated with oxygen between reduction steps. The product distribution is given in Table 4. A conversion of 71.5% and a yield of olefins and diolefins of 52.8% (carbon based) was observed, whereas the conversion and yield of pyrolysis were 55.9% and 44.8%, respectively. Performance compared to pyrolytic conversion, even with formation of CO x Higher yields can also be produced. Despite the higher conversion to unsaturated hydrocarbons, less hydrogen was observed at the outlet of the oxycrackin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com