A kind of preparation method of difluoromethoxy multi-substituted nitrogen-containing heterocyclic compound
A technology of flanges and reactors, applied in chemical instruments and methods, chemical/physical processes, chemical/physical/physical-chemical processes, etc., can solve problems such as difficult to achieve high-speed stirring, device collapse, center of gravity offset, etc., to achieve Good heating/cooling effect, stable stirring, and pressure-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
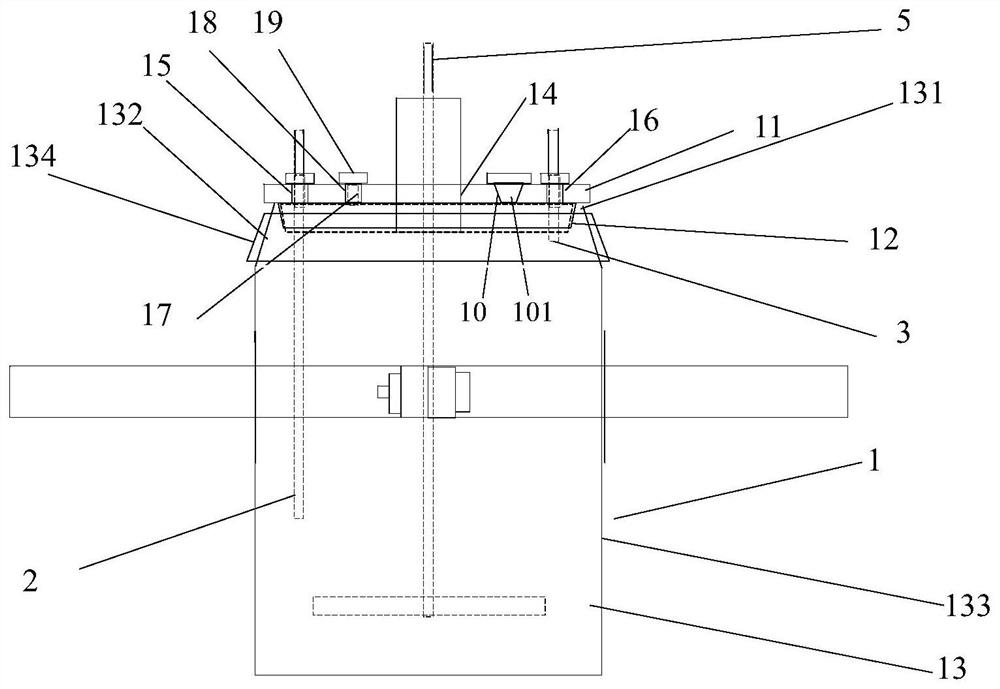
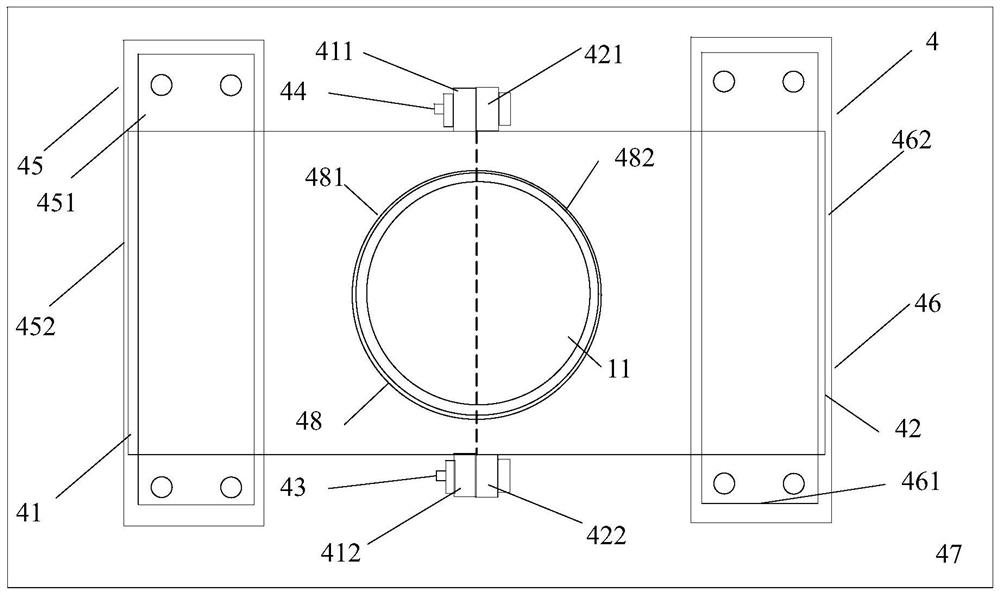
[0040] A reaction kettle, which has a body 1 made of glass, the body is equipped with a kettle cover 11, a lower flange 12, and a cylinder body 13. It is characterized in that: the reaction kettle also includes an air inlet combination 2, an air outlet combination 3, and a fixing device 4. Stirring section 5. Cooling liquid tank. The reaction kettle has improved the overall problems of the current four-neck bottle system as a whole: it is not stable enough, the bottom contact area is small, the heating and cooling are slow, the stirring speed cannot be increased, the nitrogen protection effect is not good, and the feeding must be completely opened, and it is very difficult to remove the reaction mixture. Troublesome and difficult to solve the problem.
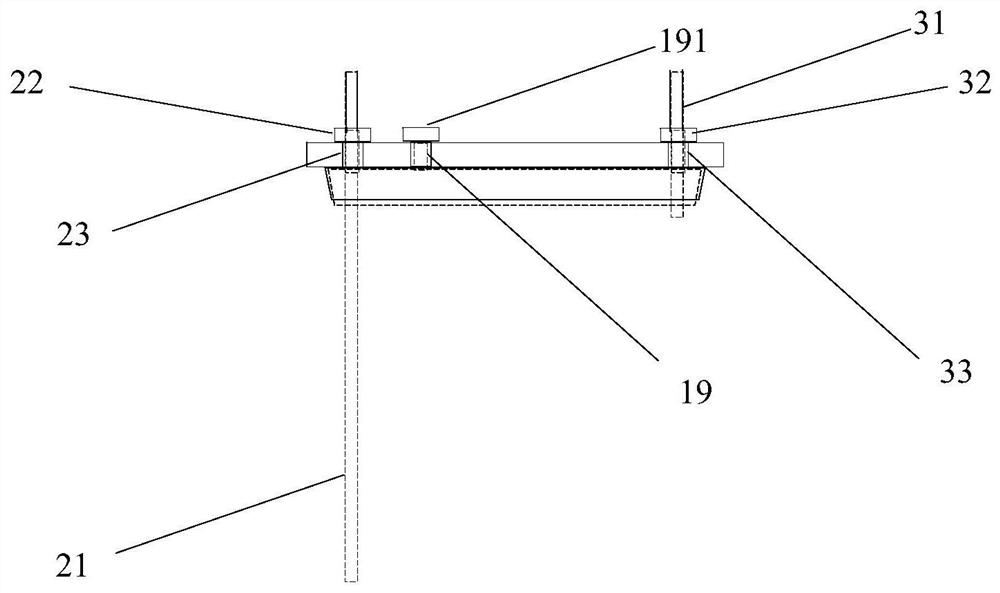
[0041] The center of kettle lid 11 has middle hole 14, and middle hole 14 is the circular through hole that the center is positioned at kettle lid 11 centers, and its inner side surface is frosted shape, is used for bonding wit...
Embodiment 2
[0052] 1) Get a reaction kettle as claimed in claim 2, wash and dry, fix the body (1) on the fixture (4), open the lid (11), add 350ml of tetrahydrofuran, and then add 70g of diisopropylamine , close the kettle cover (11), feed nitrogen gas at a speed of 140ml / min, and after 5 minutes, set the cooling liquid tank (6) in the cooling liquid circulation tank on the low-temperature cooling liquid circulation device, and make the body (1 ) is supported by the middle part of the middle tank, and an appropriate amount of cooling liquid is filled in the cooling liquid circulation tank, and the temperature is lowered to about minus 35°C. With continuous stirring at a speed of 80 rpm, the cooling liquid is slowly dripped from the feeding hole (18). Add 2.2eq of n-butyllithium, close the feed hole (18) after the dropwise addition, raise the temperature to about minus 25°C, continue stirring for 45min, cool down to minus 78°C, and drop in slowly from the feed hole (18). Contain the THF so...
Embodiment 3
[0059] 1) Get a reaction kettle as claimed in claim 2, wash and dry, fix the body (1) on the fixture (4), open the lid (11), add 360ml of tetrahydrofuran, and then add 72g of diisopropylamine , close the kettle cover (11), feed nitrogen gas at a speed of 150ml / min, and after 5 minutes, set the cooling liquid tank (6) in the cooling liquid circulation tank on the low-temperature cooling liquid circulation device, and make the body (1 ) is supported by the middle part of the middle tank, and an appropriate amount of cooling liquid is filled in the cooling liquid circulation tank, and the temperature is lowered to about minus 38°C. While stirring at a speed of 100 r / min, drip from the feeding hole (18) at a slow speed Add 2.2eq of n-butyllithium, close the feed hole (18) after the dropwise addition, raise the temperature to about minus 27°C, continue stirring for 55min, cool down to minus 79°C, and drop in slowly from the feed hole (18) Contain the THF solution of the 2-bromo-3-f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com