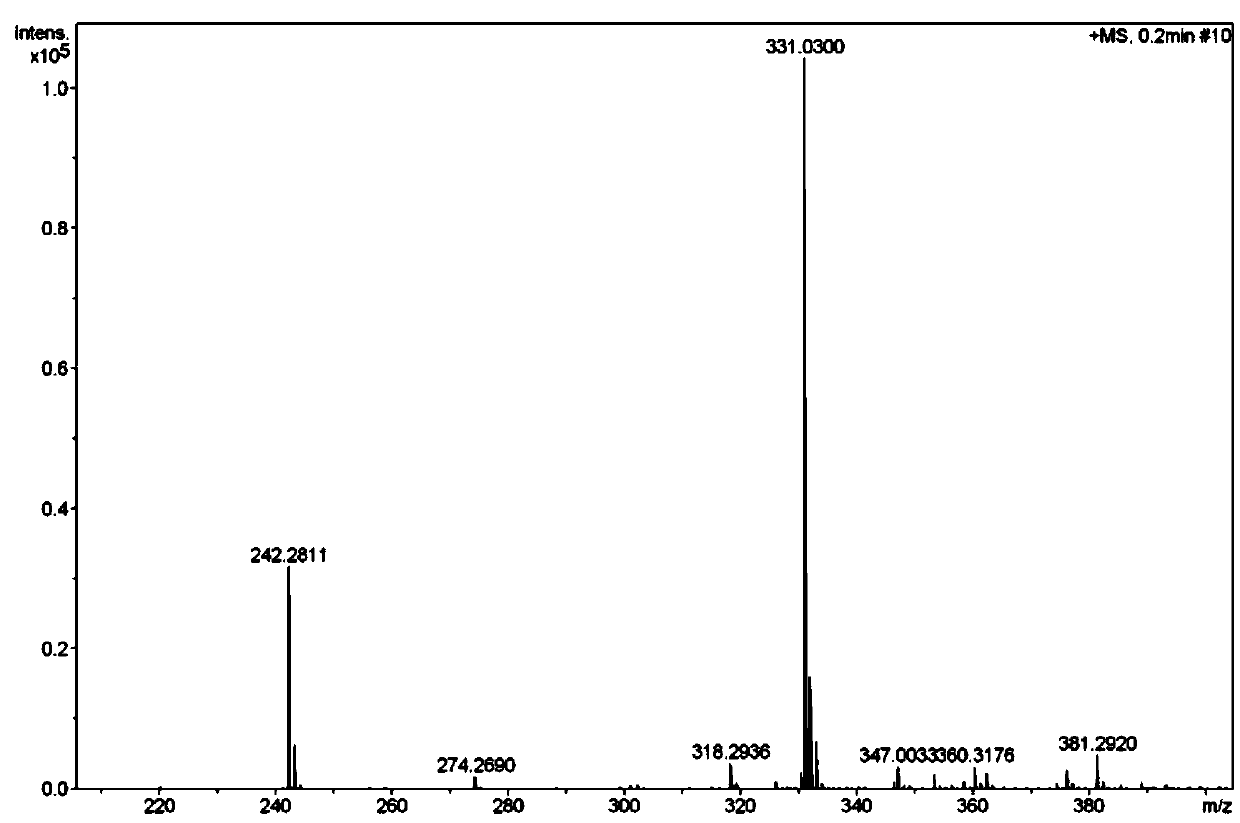
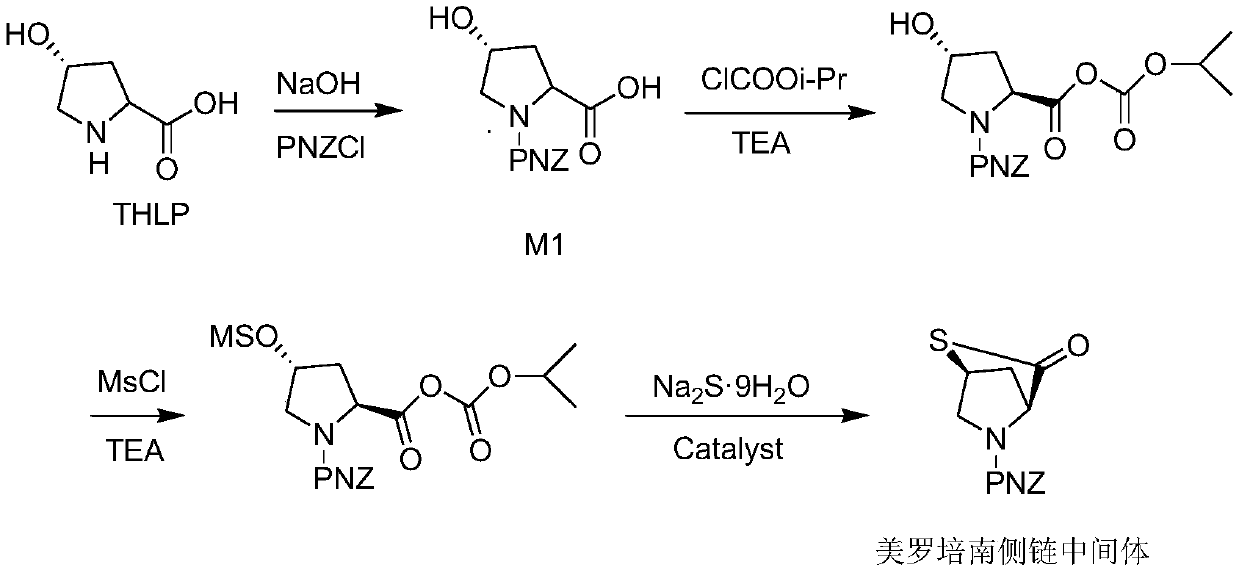
Preparation method of meropenem side chain intermediate mercaptan lactone
A technology of meropenem side chains and body thiols, which is applied in the field of chemical substance preparation, can solve problems such as low yield, high production cost, and long synthetic route, and achieve the effects of high purity, low cost, and improved conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Add 1.8 g of M1 ([(2S,4R)-2-carboxy-1-(4-nitrobenzyloxycarbonyl)pyrrolidine)] to 31 mL of dichloromethane, the same below), and keep the liquid temperature at -26 ℃ first drop 0.71g isopropyl chloroformate, then drop 0.78g TEA, react for 15min;
[0030] 2) Add 0.86g MsCl dropwise at -26°C, then add 0.71g TEA dropwise, and react for 26min;
[0031] 3) Add 1.81g Na 2 S·9H 2 O. Dissolve 0.24g of polyethylene glycol 600 and 1.74g of water into a solution, add this solution into the reaction solution at -26°C, raise the temperature from -26°C to 0°C, and keep the heating time for 26min;
[0032]4) After the temperature rise, add deionized water at 0°C for liquid separation, separate the organic phase, raise the temperature of the organic phase to 40°C for reflux reaction, and reflux for 2.5 hours. After the reaction is completed, add 5% Adjust the potassium carbonate solution to pH=9, separate the oil phase 1, continue to adjust the oil phase 1 to pH=1 with 18% hydroch...
Embodiment 2
[0034] 1) Add 1.8g of M1 to 32mL of dichloromethane, keep the liquid temperature at -24°C, add 0.71g of isopropyl chloroformate dropwise, then add 0.77g of TEA dropwise, and react for 19min;
[0035] 2) Add 0.80g MsCl dropwise at -24°C, then add 0.65g TEA dropwise, and react for 30min;
[0036] 3) Add 1.67g Na 2 S·9H 2 O, 0.24g of 18-crown-6 and 1.74g of water were dissolved into a solution, and this solution was added to the reaction solution at -24°C, and the temperature was raised from -24°C to 0°C, and the heating time was maintained for 22 minutes;
[0037] 4) After the temperature rise, add deionized water at 0°C for liquid separation, separate the organic phase, raise the temperature of the organic phase to 38°C for reflux reaction, and reflux for 2.6 hours. After the reaction is completed, add 4% Adjust the potassium carbonate solution to pH=8.3, separate the oil phase 1, and continue to adjust the oil phase 1 to pH=1.1 with 17% hydrochloric acid solution at -1°C, se...
Embodiment 3
[0039] 1) Add 1.8g M1 to 33mL dichloromethane, keep the liquid temperature at -20°C, add 0.85g isopropyl chloroformate dropwise, then add 0.82g TEA dropwise, and react for 17min;
[0040] 2) Add 0.93g MsCl dropwise at -20°C, then add 0.77g TEA dropwise, and react for 22min;
[0041] 3) Add 1.65g Na 2 S·9H 2 O. Dissolve 0.24g tetradecyltrimethylammonium chloride and 1.74g water into a solution, add this solution into the reaction solution at -20°C, raise the temperature from -20°C to 0°C, and keep the heating time for 30min;
[0042] 4) After heating up, add deionized water at 0°C for liquid separation, separate the organic phase, raise the temperature of the organic phase to 39°C for reflux reaction, the reflux reaction time is 2.7h, after the reaction is completed, add 3% carbonic acid at 0°C Adjust the potassium solution to pH=8.7, separate the oil phase 1, continue to adjust the oil phase 1 to pH=1.6 with 16% hydrochloric acid solution at 0°C, separate the oil phase 2, di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com