Benzophenone derivative photoinitiator and preparation method thereof
A technology of benzophenones and photoinitiators, which is applied in the preparation of sulfides, carboxylic acid halides, organic chemistry, etc., can solve the problems of easy migration of photoinitiators, and achieve the reduction of migration, increase of molecular weight and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The structural formula of compound 1:
[0025]
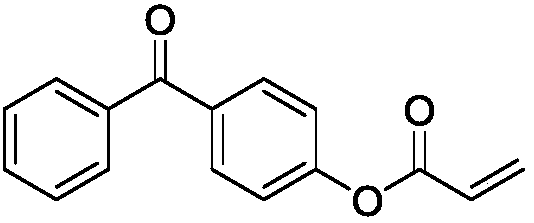
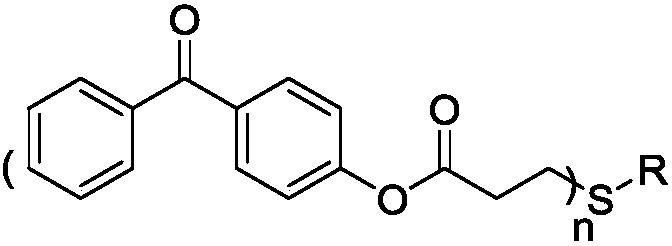
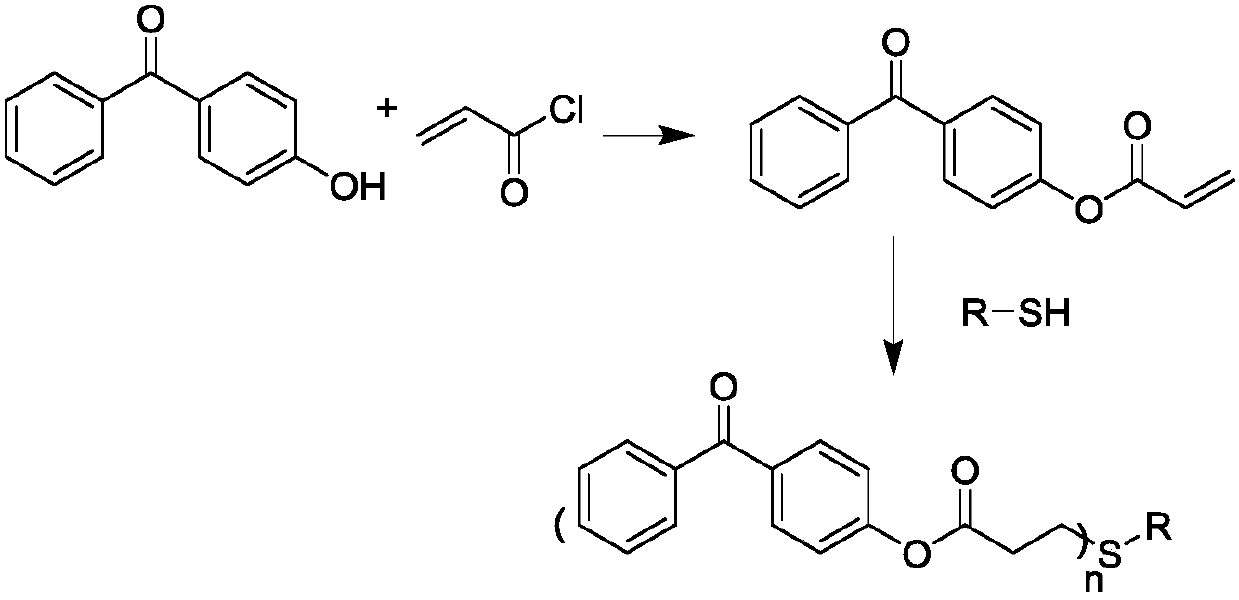
[0026] 4-Hydroxybenzophenone (19.8g, 0.1mol), triethylamine (12.12g, 0.12mol) and 100ml of methylene chloride were added to a 250ml three-neck flask equipped with mechanical stirring and a constant pressure dropping funnel, Stir for 5 min in an ice-water bath. Acryloyl chloride (10.86 g, 0.12 mol) was added to a constant pressure dropping funnel filled with 50 ml of dichloromethane solvent, and the addition rate was controlled. The addition was completed within 2 H, and finally stirred at room temperature for 6 H. After the reaction was completed, remove solid impurities by filtration, and deionized water, HCl (1mol / L) and NaHCO 3 (1mol / L) aqueous solution was washed twice, the organic layer was dried and filtered, and the solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized with ethanol to obtain an intermediate product A. Subsequently, the intermediate product A and methyl 3...
Embodiment 2
[0029] The structural formula of compound 2 is:
[0030]
[0031] 4-Hydroxybenzophenone (19.8g, 0.1mol), triethylamine (12.12g, 0.12mol) and 100ml of methylene chloride were added to a 250ml three-neck flask equipped with mechanical stirring and a constant pressure dropping funnel, Stir for 5 min in an ice-water bath. Acryloyl chloride (10.86 g, 0.12 mol) was added to a constant pressure dropping funnel filled with 50 ml of dichloromethane solvent, and the addition rate was controlled. The addition was completed within 2 H, and finally stirred at room temperature for 6 H. After the reaction was completed, remove solid impurities by filtration, and deionized water, HCl (1mol / L) and NaHCO 3 (1mol / L) aqueous solution was washed twice, the organic layer was dried and filtered, and the solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized with ethanol to obtain an intermediate product A. Subsequently, intermediate product A and 2,3-dimer...
Embodiment 3
[0034] The structural formula of compound 3 is:
[0035]
[0036] 4-Hydroxybenzophenone (19.8g, 0.1mol), triethylamine (12.12g, 0.12mol) and 100ml of methylene chloride were added to a 250ml three-neck flask equipped with mechanical stirring and a constant pressure dropping funnel, Stir for 5 min in an ice-water bath. Acryloyl chloride (10.86 g, 0.12 mol) was added to a constant pressure dropping funnel filled with 50 ml of dichloromethane solvent, and the addition rate was controlled. The addition was completed within 2 H, and finally stirred at room temperature for 6 H. After the reaction was completed, remove solid impurities by filtration, and deionized water, HCl (1mol / L) and NaHCO 3 (1mol / L) aqueous solution was washed twice, the organic layer was dried and filtered, and the solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized with ethanol to obtain an intermediate product A. Subsequently, intermediate product A and trimethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com