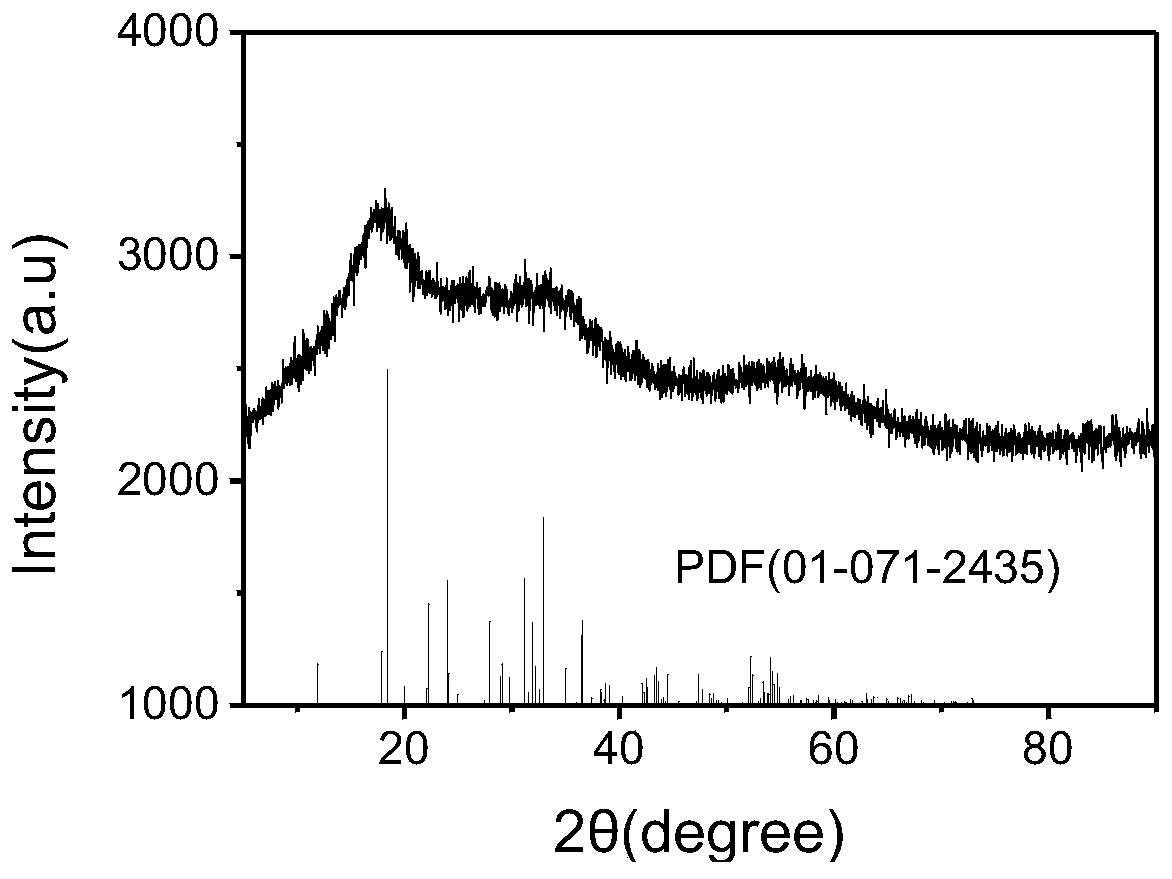
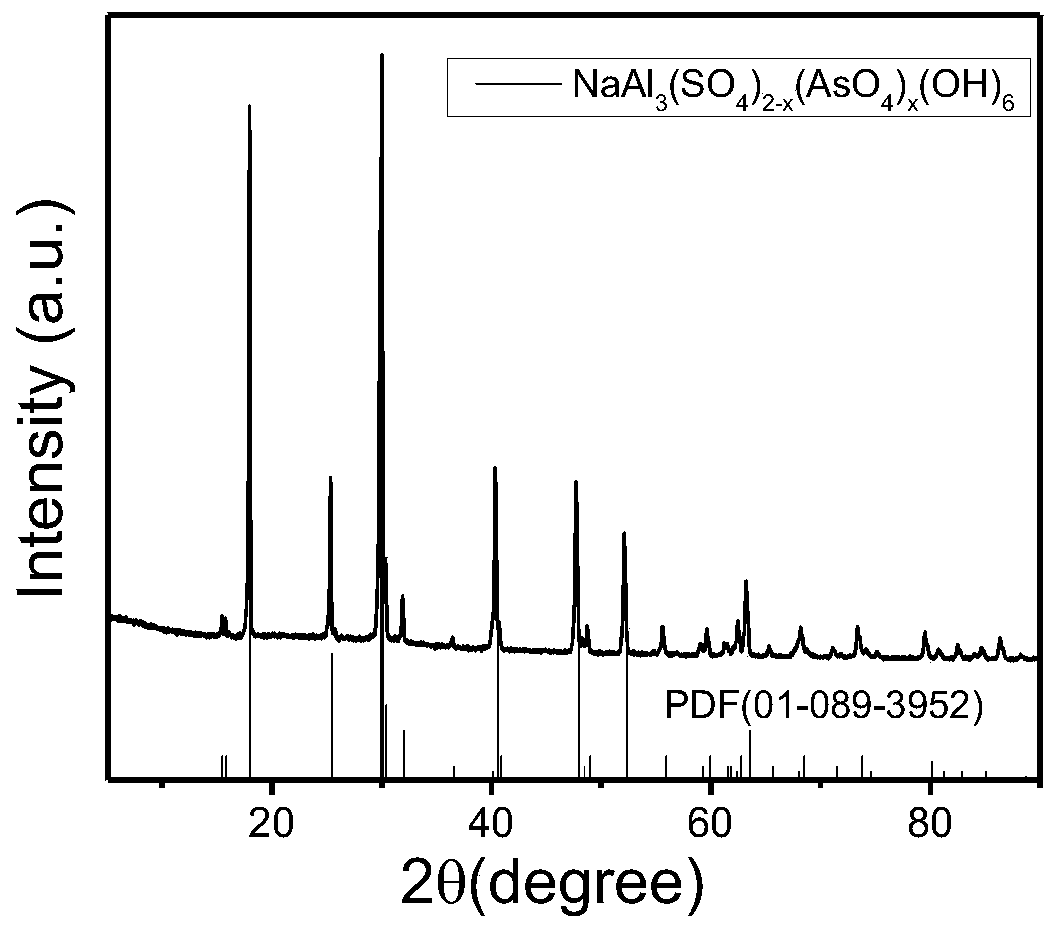
Method for synchronously solidifying and stabilizing arsenic sulfide residues and recycling sulfur resources
A technology for resource recovery and arsenic sulfide, applied in chemical instruments and methods, preparation/purification of aluminum-sulfur compounds, sulfur, etc., can solve problems such as poor long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0028] Take 0.1g of slag containing simulated arsenic sulfide in the reactor, add hydrogen peroxide containing 10%, aluminum sulfate, sodium sulfate concentration is respectively 0.08mol / L, the mixed solution of 0.08mol / L, make solid-liquid ratio be 1: 150g / ml, adjust pH=2, stir evenly, put the reaction kettle at 200°C for 2h, and cool down to room temperature naturally. Through cleaning and flotation, yellow agglomerated sulfur and sodium arsenic alunite solids are finally separated, the recovery rate of sulfur reaches 90%, and its purity exceeds 95%; the mass fraction of its arsenic is 2.85%, and the volume of waste residue is reduced by 60%; The toxic leaching of arsenic is 0.1mg / L.
Embodiment example 2
[0030]The arsenic sulfide sludge, a product of acid wastewater treatment in a smelter in Fujian Province, was taken as the research object. The mass analysis of all elements shows that the main elements in the arsenic slag are As 53.4%, S 42%, Na 1.5%, Ni 1.3%, and Cu 0.98%. Take 0.1g of arsenic-containing waste slag in the reaction kettle and add 10% hydrogen peroxide , the concentrations of aluminum sulfate and sodium sulfate are 0.08mol / L and 0.08mol / L respectively, so that the solid-liquid ratio is 1:150g / ml, adjust the pH=2, stir evenly, and put the reaction kettle at 200°C Reaction 2h, naturally cooled to room temperature. Through cleaning and flotation, yellow agglomerated sulfur and arsenic sodium alunite solids are finally separated, the recovery rate of sulfur reaches 90%, and its purity exceeds 95%; the mass fraction of its arsenic is 2.38%, and the volume of waste residue is reduced by 60%; The toxic leaching of arsenic is 0.16mg / L.
Embodiment example 3
[0032] The arsenic sulfide slag produced after sulfidation and precipitation of polluted acid wastewater from a non-ferrous metal smelter in Henan Province was taken as the research object. The mass analysis of all elements shows that the main elements in the arsenic slag are As 11.35%, S 37.96%, O 26.09%, Cu 23.53%, and Al 1.07%. Take 0.2g of arsenic-containing waste slag in the reactor and add 10% peroxide The concentration of hydrogen, aluminum sulfate, and sodium sulfate is 0.08mol / L, 0.08mol / L mixed solution, so that the solid-liquid ratio is 1:80g / ml, adjust pH = 2, after stirring evenly, put the reaction kettle at 200°C Under the reaction 2h, naturally cooled to room temperature. Through cleaning and flotation, yellow agglomerated sulfur and sodium arsenic alunite solids are finally separated, the recovery rate of sulfur reaches 90%, and its purity exceeds 96%; the mass fraction of its arsenic is 5.64%, and the volume of waste residue is reduced by 60%; The toxic leach...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com