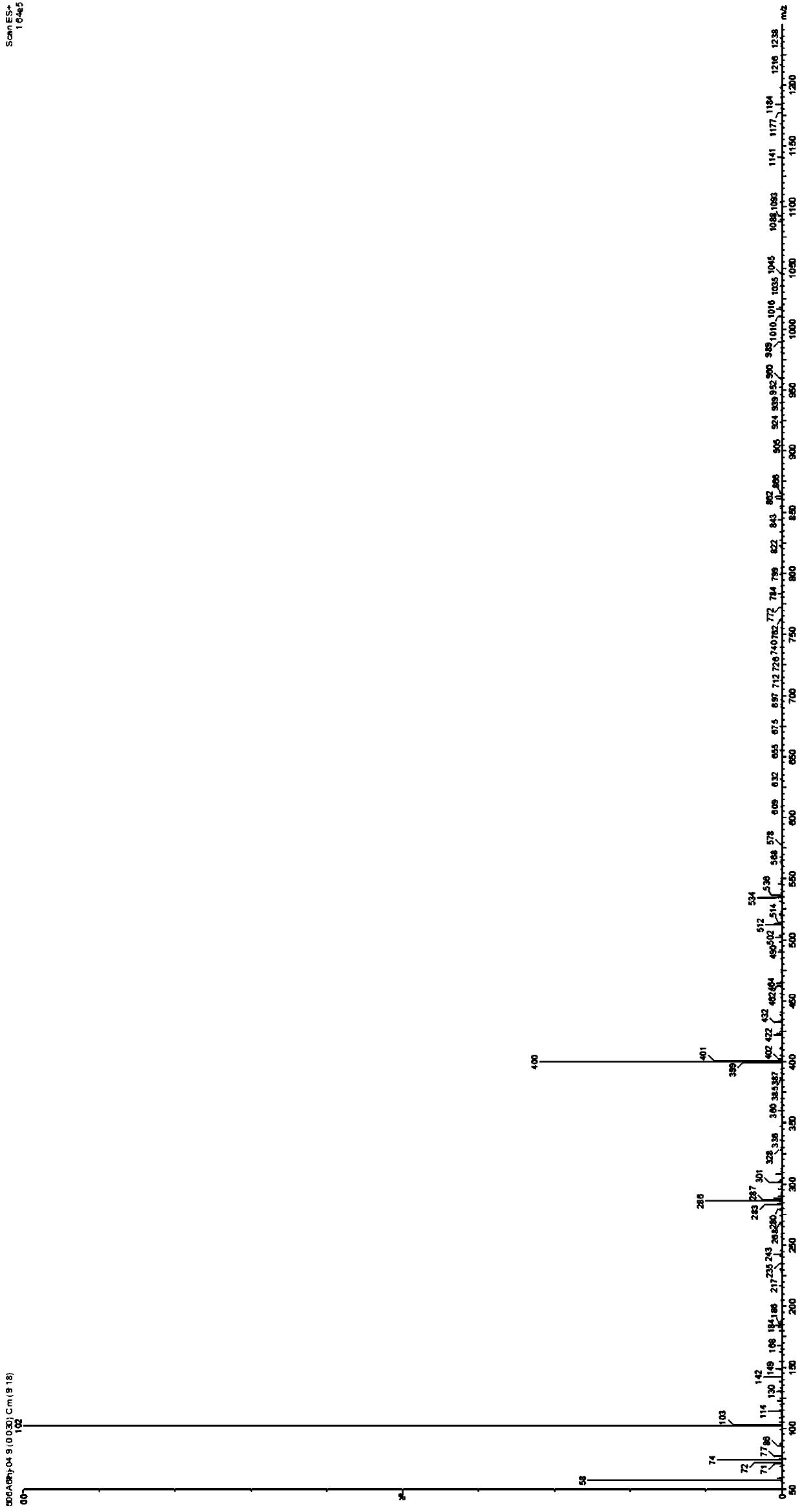
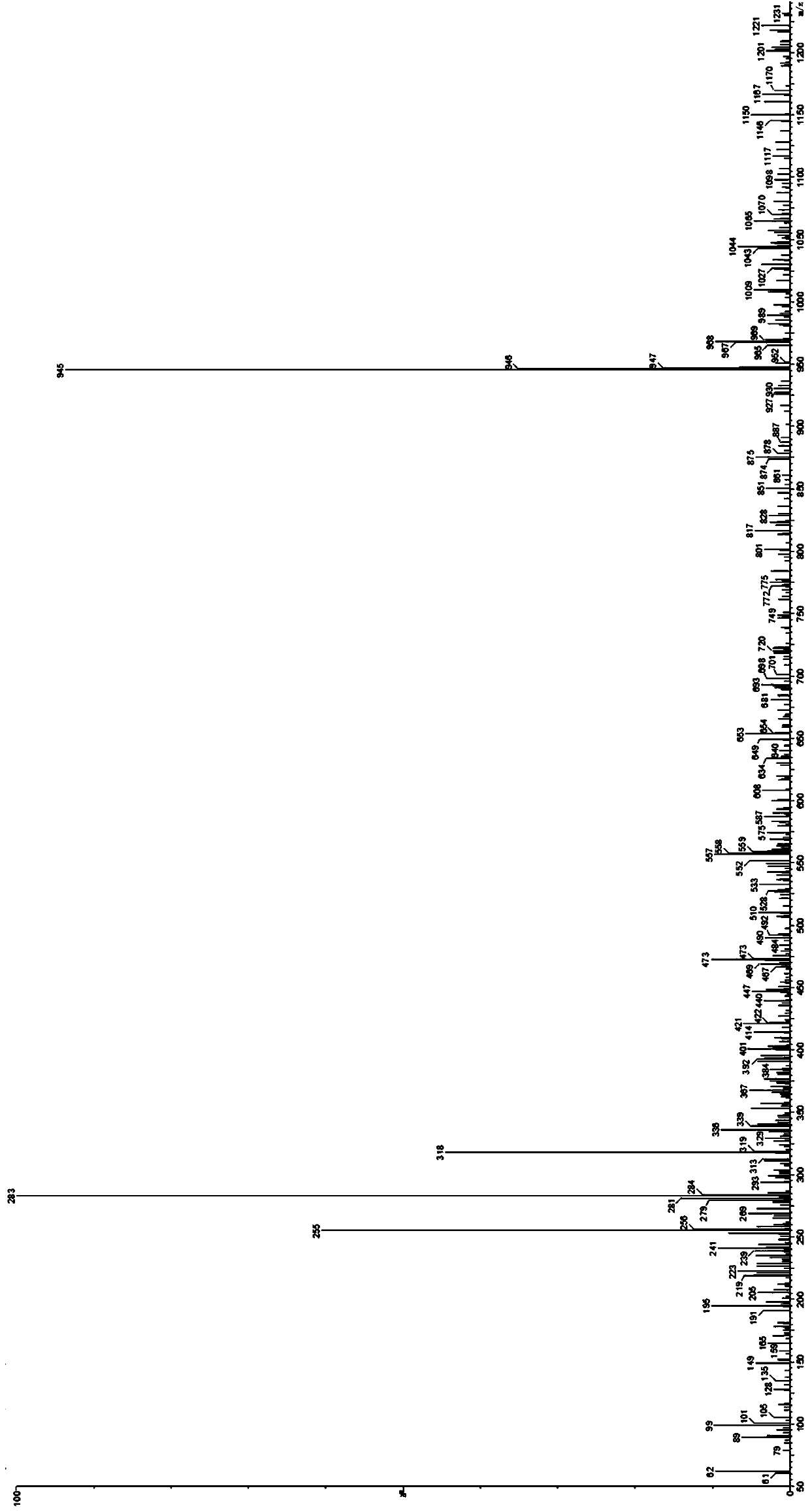
Nucleic acid gel electrophoresis testing method
A nucleic acid gel and electrophoresis technology, applied in the field of nucleic acid gel electrophoresis testing, can solve the problems of inability to guarantee the true level of DNA migration, low toxicity, and insufficient sensitivity, etc., and achieve simple and controllable preparation methods, stable molecular structure, and high sensitivity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027]
[0028]
[0029]
[0030]
[0031]
[0032] Put 60.0g 3,8-diamino-6-phenylphenanthridine, 360ml DMF and 37.2ml Pyridine in a 1000ml three-neck round bottom flask, stir mechanically, cool to 0-5°C in an ice-water bath; add 42ml II dropwise, drop Keep warm at 0-5°C during the addition process; continue to react for ten hours until the reaction is completed; filter with suction, add the filter cake to about 2L of pure water, stir mechanically for 30 minutes, and filter with suction; wash the filter cake with 2L of pure water again, Suction filtration to dryness; drying under reduced pressure to constant weight to obtain 48 grams of yellow solid; weigh 347 g of ethyl 6-iodohexanoate, put it in a 2000 ml three-necked flask, add 39.4 g of intermediate I under mechanical stirring, and heat in an oil bath to 100-110°C, react for 3 days until the reaction is complete; cool down to 80°C, add 1500mlEA, reflux for 1h, stop heating, and naturally cool down...
Embodiment 2
[0034]
[0035]
[0036]
[0037]
[0038]
[0039]
[0040]
[0041] In a 100 mL three-necked flask equipped with a reflux condenser, add 40 mL of chlorobenzene, and add SM (10 g) into it while stirring. Add dimethyl sulfate (4 mL), mix the three, raise the temperature to reflux state, TLC tracking, developer: methanol (5%) ethyl acetate (95%) and add a small amount of acetic acid, until the end of the reaction (about 2-3 Hour). Add 10 mL of ethanol and continue to reflux for 15 min, stop heating and cool to room temperature, directly filter to remove the solvent, wash the filter cake with ether 2-3 times, and dry the solid in vacuum to obtain the product intermediate I (8 g).
[0042] Add Intermediate I (7 g), 100 mL of acetonitrile, and 20 mL of water to a 250 mL three-necked flask equipped with mechanical stirring and a reflux condenser, gradually heat to reflux, and then reduce iron powder (15 g) and trichloride Iron (200 mg) was added into the reactio...
Embodiment 3
[0047]
[0048]
[0049]
[0050]
[0051]
[0052]
[0053]
[0054]
[0055]
[0056] Add 2-amino-4,4'-dinitrobiphenyl (17.2 g) and 3-cyanobenzoyl chloride (11 g) into a 250 mL three-necked flask equipped with a reflux condenser, followed by 60 mL of chlorobenzene , stirred by magnetic force and gradually heated to reflux state, and reacted for 4 hours. After the reaction was completed, it was cooled to room temperature, and the chlorobenzene was directly removed by filtration. The obtained filter cake was recrystallized and purified with acetic acid, and the obtained solid was vacuum-dried and dried to obtain the product intermediate I (24.1 g).
[0057] Add Intermediate I (18 g) and 60 mL of nitrobenzene to a 250 mL single-necked flask equipped with a reflux condenser, and add 6 mL of POCl under magnetic stirring 3 , gradually heated to 200°C for 3 hours, then cooled to room temperature, and nitrobenzene and POCl were distilled off under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com