Proton conductor fuel cell cathode material with B-site defect, electrolyte and preparation method thereof
A fuel cell cathode and fuel cell technology, applied in the direction of fuel cells, battery electrodes, material separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
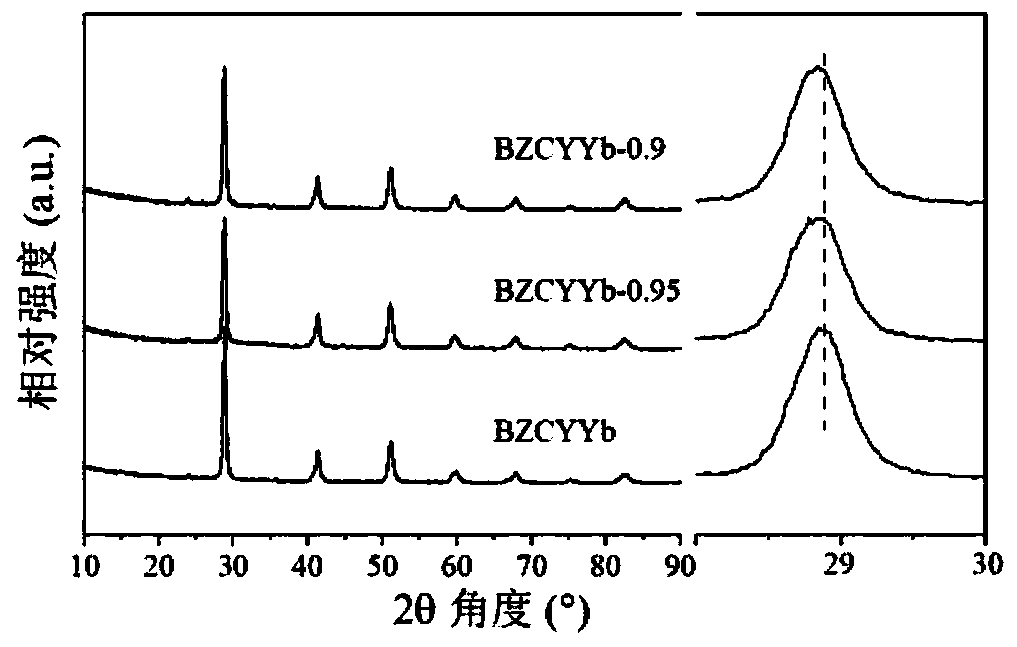
[0072] This embodiment provides a medium and low temperature solid oxide fuel cell electrolyte material Ba(Zr 0.1 Ce 0.7 Y 0.1 Yb 0.1 ) 0.95 o 3-δ The preparation method, concrete steps are as follows:
[0073] (1) Weigh 2.7441 g of barium nitrate, 0.4293 g of zirconium nitrate, 3.0395 g of cerium nitrate, 0.3830 g of yttrium nitrate and 0.4491 g of ytterbium nitrate, add a small amount of deionized water to dissolve. Weigh 30 g of ethylenediamine tetraacetic acid and 42 g of citric acid hydrate as a complexing agent and dissolve them in deionized water at a molar ratio of EDTA:citric acid hydrate:total metal ions of 1:2:1.
[0074] (2) After adding the solution containing complexing agent to the solution containing metal ions, add an appropriate amount of ammonia water dropwise to bring the pH of the solution to 7-8, and then stir under the condition of magnetic stirring to completely evaporate the water to obtain a gel substance.
[0075] (3) The gel-like substance wa...
Embodiment 2
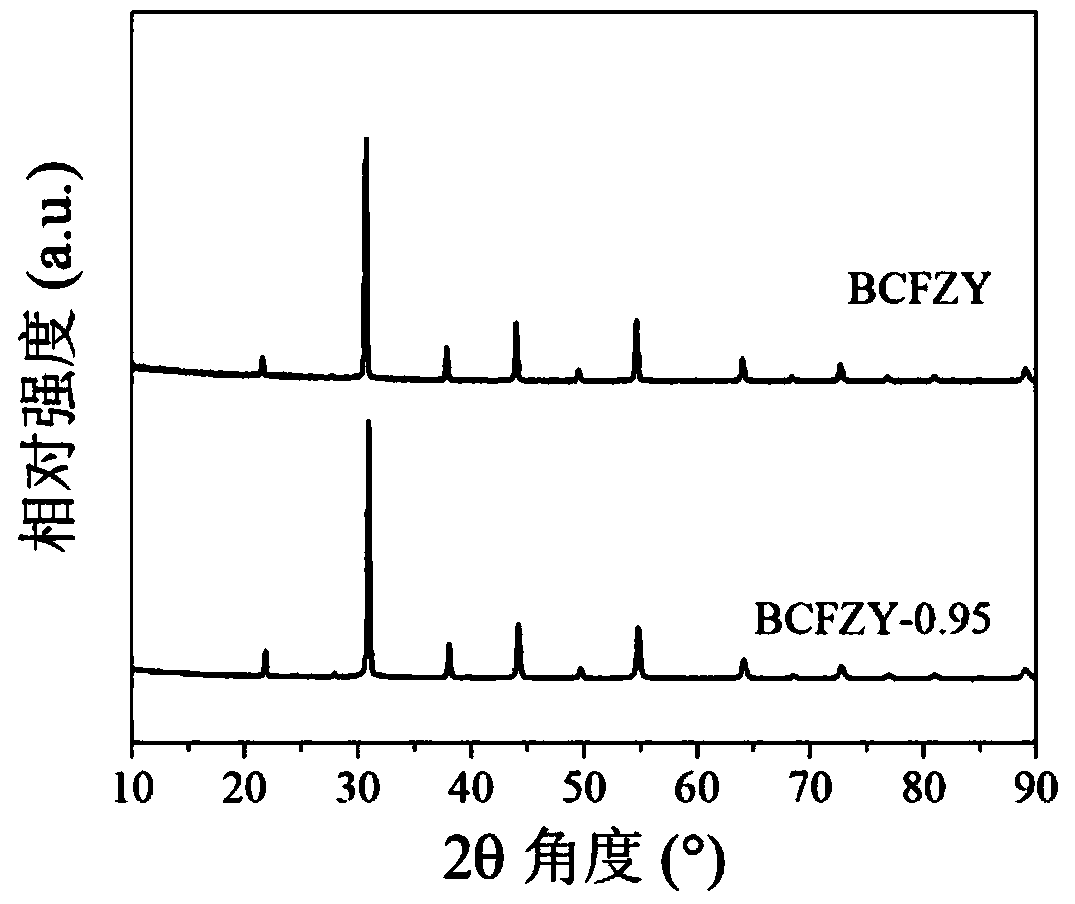
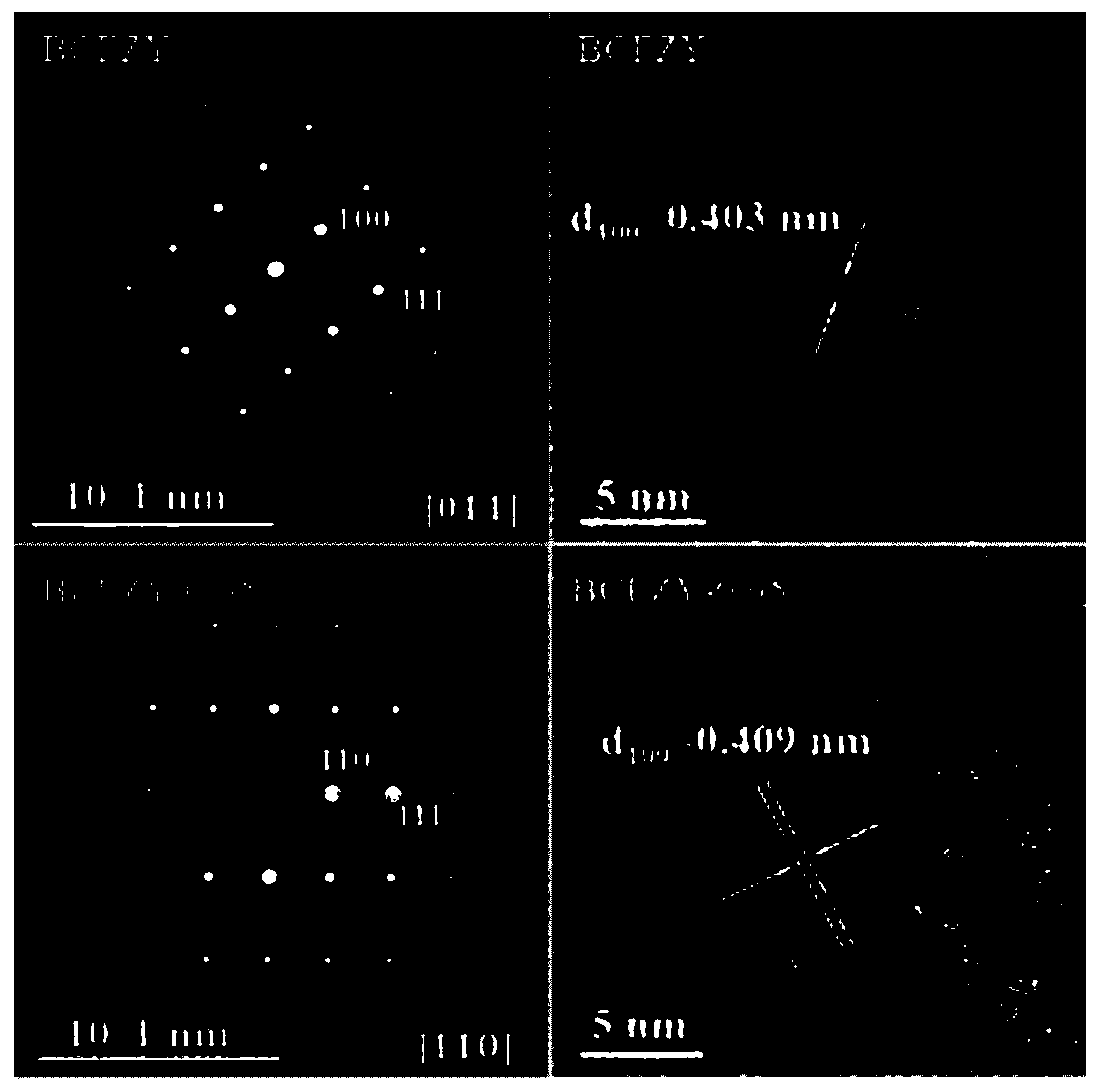
[0078] This embodiment provides a medium and low temperature solid oxide fuel cell cathode powder Ba(Co 0.4 Fe 0.4 Zr 0.1 Y 0.1 ) 0.95 o 3-δ The preparation method, concrete steps are as follows:
[0079] (1) Weigh 13.7204 g of barium nitrate, 5.8206 g of cobalt nitrate, 8.08 g of ferric nitrate, 2.1466 g of zirconium nitrate and 1.9151 g of yttrium nitrate, add a small amount of deionized water to dissolve. Weigh 30 g of ethylenediamine tetraacetic acid and 42 g of citric acid hydrate as a complexing agent and dissolve them in deionized water at a molar ratio of EDTA:citric acid hydrate:total metal ions of 1:2:1.
[0080] (2) After adding the solution containing complexing agent to the solution containing metal ions, add an appropriate amount of ammonia water dropwise to bring the pH of the solution to 7-8, and then stir under the condition of magnetic stirring to completely evaporate the water to obtain a gel substance.
[0081] (3) The gel-like substance was placed i...
Embodiment 3
[0084] This embodiment specifically provides a kind of Ba(Co 0.4 Fe 0.4 Zr 0.1 Y 0.1 ) 0.95 o 3-δ The test for preparing symmetrical battery polarization impedance for the battery cathode includes the following specific steps:
[0085] (1) Weigh 1 g of the cathode powder Ba(Co 0.4 Fe 0.4 Zr 0.1 Y 0.1 ) 0.95 o 3-δ , 10 ml of isopropanol, 2 ml of ethylene glycol, and 0.8 ml of glycerol were poured into a high-energy ball mill, and after ball milling at 400 r / min for 30 min, they were transferred to the strain bottle with a straw to obtain the desired cathode slurry.
[0086] (2) Place the prepared BZCYYb-0.95 electrolyte on a heating table to preheat at 200°C, use a spray gun to spray the prepared cathode slurry evenly on both sides of the electrolyte under the push of an inert gas, and wait for the liquid to evaporate completely Finally, the sprayed electrolyte was placed in a high-temperature muffle furnace and calcined at 900 °C for 2 h to prepare the desired symm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com