Preparation and application of MOF-supported bimetallic catalyst
A catalyst and bimetallic technology, which is applied in the field of preparation of MOF-supported bimetallic catalysts, can solve the problems of impaired stability, need to improve stability, and reduced activity, and achieve the effects of low stability, stable activity, and difficulty in agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
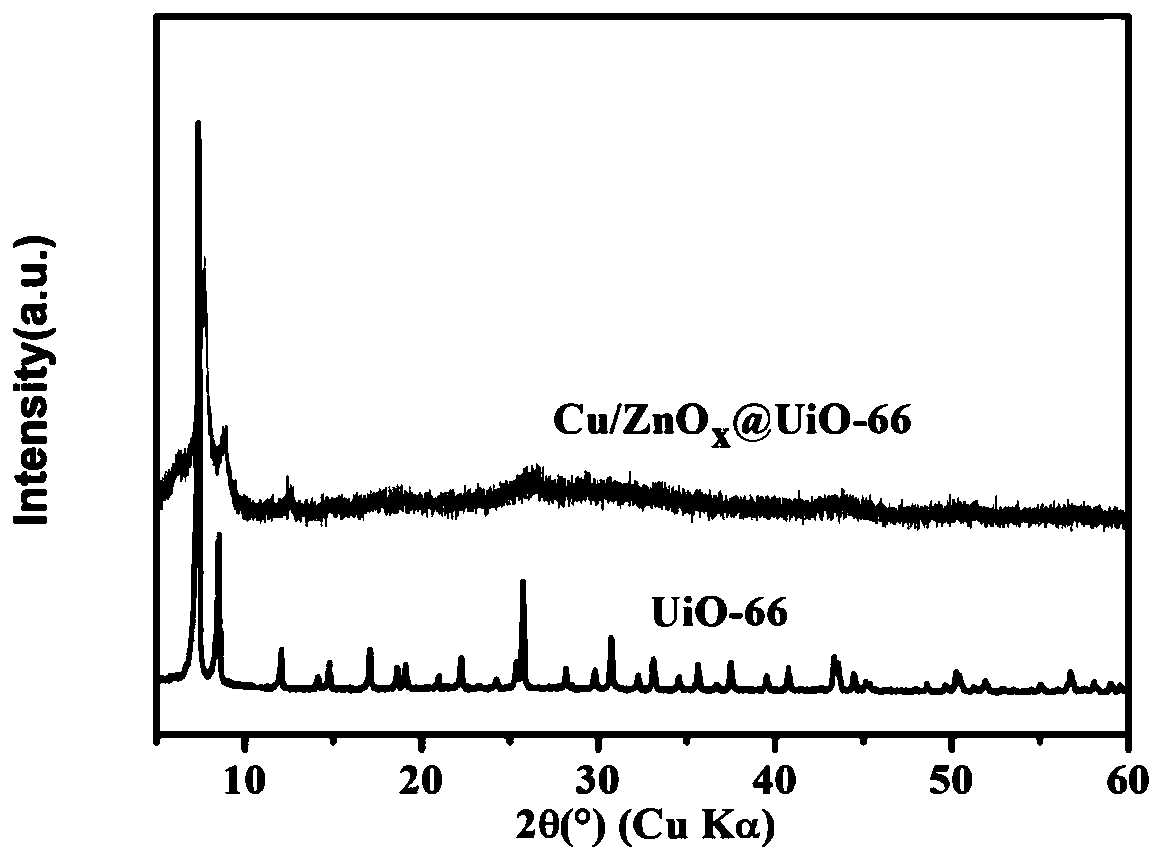
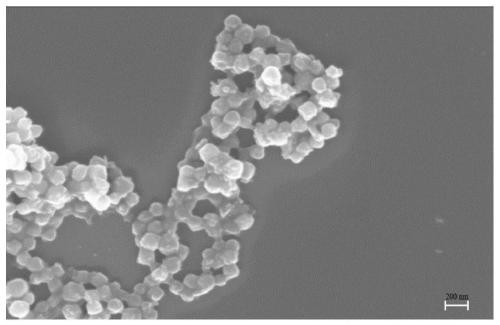
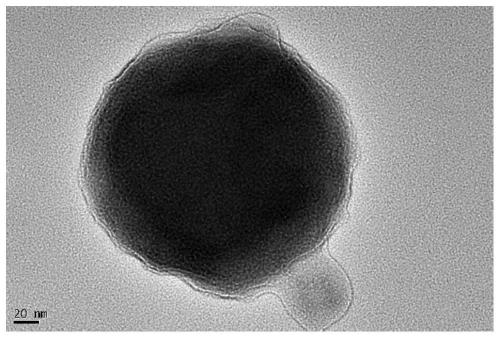
[0032] CuZnO x Preparation and application of @UiO-66 catalyst.
[0033] MOF (UiO-66) can be purchased commercially or synthesized by itself. The preparation method of MOF (UiO-66) in this example is as follows:
[0034] Add 0.912g of terephthalic acid, 1.280g of zirconium chloride, 320mL of N,N-dimethylformamide (DMF), and 14mL of glacial acetic acid into a hydrothermal kettle, react at a temperature of 393.25K for 24h, and take out the natural cool down. After cooling to room temperature, it was centrifuged to obtain a white solid. The product was washed more than 3 times with DMF, and after removing excess reactants, solvent replacement was performed with ethanol. The replacement followed a small number of times, 30mL to 50mL of ethanol each time, and replaced once every 24h. After more than 3 replacements, vacuum drying was performed to obtain a white solid powder MOF (UiO-66).
[0035] CuZnO x The preparation method of @UiO-66 catalyst is as follows:
[0036] 1) Wei...
Embodiment 2
[0047] First, disperse 1.0g of carrier MIL-100 in 50mL of dichloromethane, ultrasonically and stir until uniformly dispersed, and at the same time prepare 1mL of copper nitrate aqueous solution with a concentration of 0.50g / mL, and slowly add it dropwise into dichloromethane, and stir for 12h , and Cu was obtained after vacuum drying 2+ @MIL-101. Then, the Cu 2+ @MIL-101 placed in a fixed bed reactor with 5% H 2 -Ar mixed gas was reduced at 250°C to obtain Cu@MIL-101. Finally, the prepared Cu@MIL-101 was added to 50 mL of tetrahydrofuran, ultrasonically and stirred until uniformly dispersed, then 0.5 g of platinum acetylacetonate was added, stirred for 12 hours under an inert atmosphere, centrifuged, washed with THF for 3 times, vacuum Cu / PtO after drying x @MIL-101.
[0048] The Cu / PtO@MIL-101 was loaded into the fixed bed reactor, and the 2 After reduction, change the reaction gas (H 2 / CO 2 / N 2 ), adjust the temperature, gas velocity, and pressure to carry out the...
Embodiment 3
[0050] First, disperse 1.0g of carrier MIL-101 in 50mL of carbon tetrachloride, ultrasonically and stir until uniformly dispersed, and at the same time prepare 1mL of platinum chloride aqueous solution with a concentration of 0.10g / mL, and slowly add it dropwise into carbon tetrachloride , stirred for 12h, and dried under vacuum to obtain Pt 2+ @MIL-101. Then, Pt 2+ @MIL-101 placed in a fixed bed reactor with 5% H 2 -Ar mixed gas was reduced at 250°C to obtain Pt@MIL-101. Finally, the prepared Pt@MIL-101 was added to 50 mL of tetrahydrofuran, ultrasonically and stirred until uniformly dispersed, then 1.2 mL of diethylzinc in tetrahydrofuran (1 moL / L) was added under an inert atmosphere, and stirred under an inert atmosphere After 12 hours, it was separated by centrifugation, washed three times with tetrahydrofuran, and dried in vacuum to obtain Pt / ZnO@MIL-101.
[0051] Pt / ZnO x @MIL-101 is loaded into a fixed bed reactor and passed through H 2 After reduction, change the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com