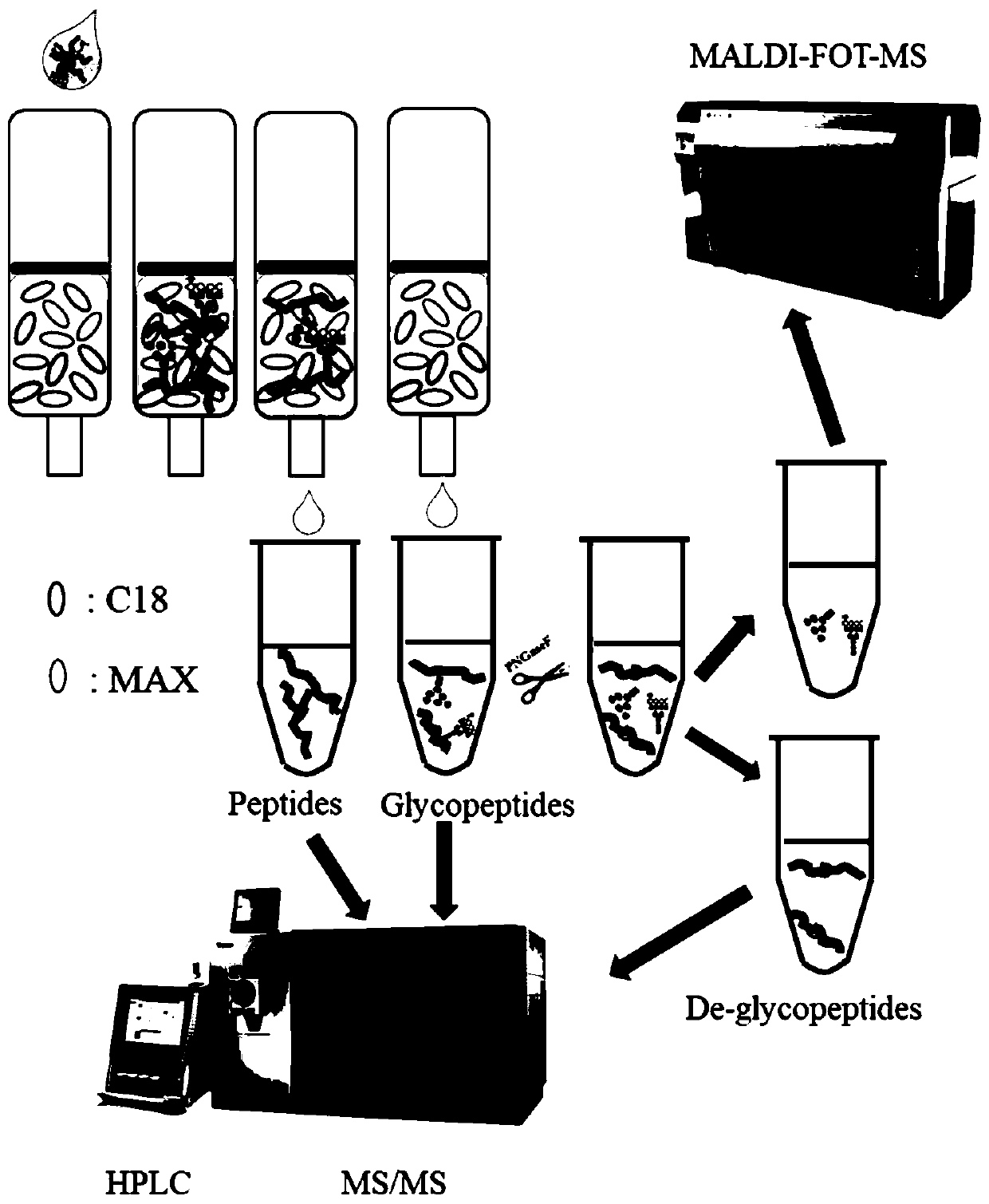
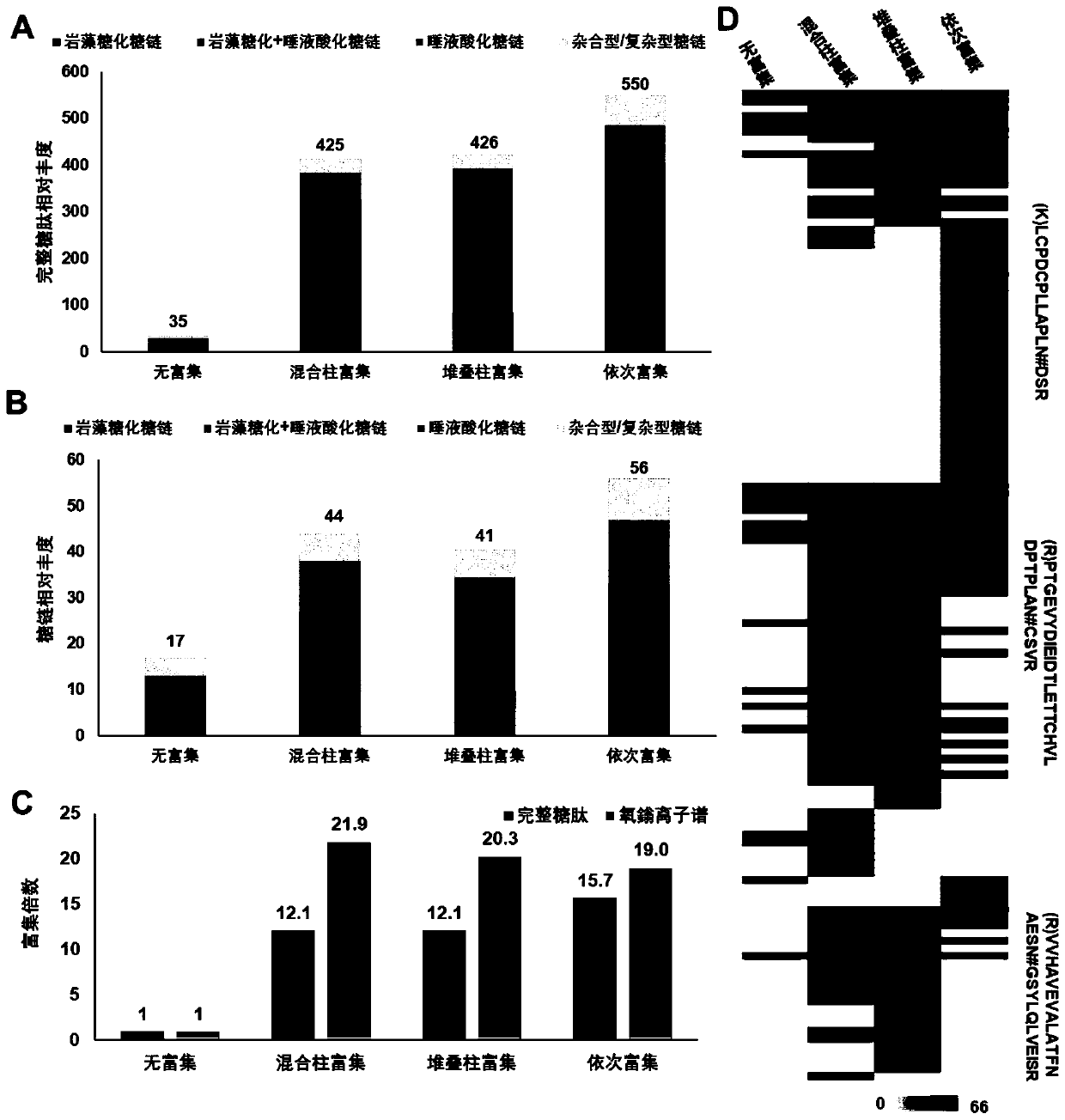
Method for rapidly analyzing protein and strong-polarity long amino acid sequence glycopeptides in biological sample
A technology for rapid analysis of biological samples, applied in the field of glycopeptide analysis, can solve the problems of complete glycopeptide analysis efficiency and coverage reduction, time-consuming, sample loss, etc., and achieve high enrichment and identification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Sample protein preparation:
[0033] Preparation of cell / tissue protein: use cell protein reagent extraction kit (such as T-PER, RIPA) to extract cell protein, try to use less lysate to ensure the protein concentration is 2-10mg / mL, and centrifuge at 14,000g for 5min to remove cell residues . The protein concentrations of the two cell extracts were determined with a BCA kit (guaranteed at least three repetitions).
[0034] Serum protein preparation: Serum samples were centrifuged at 12,000g for 15 minutes, and the middle liquid part was taken. Add to a 10KD ultrafiltration tube, place the ultrafiltration tube in a matching centrifuge tube, centrifuge at 12,000g for 15min, add 400μl of 40mM NH 4 HCO 3, centrifuge again, and repeat. Invert the filter membrane into a new centrifuge tube, centrifuge at 9000g for 3min, collect the isolated protein (about 50μl), quantify it with the Bradford method, and finally adjust the volume to 2mg / ml.
[0035] Preparation of exosome...
Embodiment 2
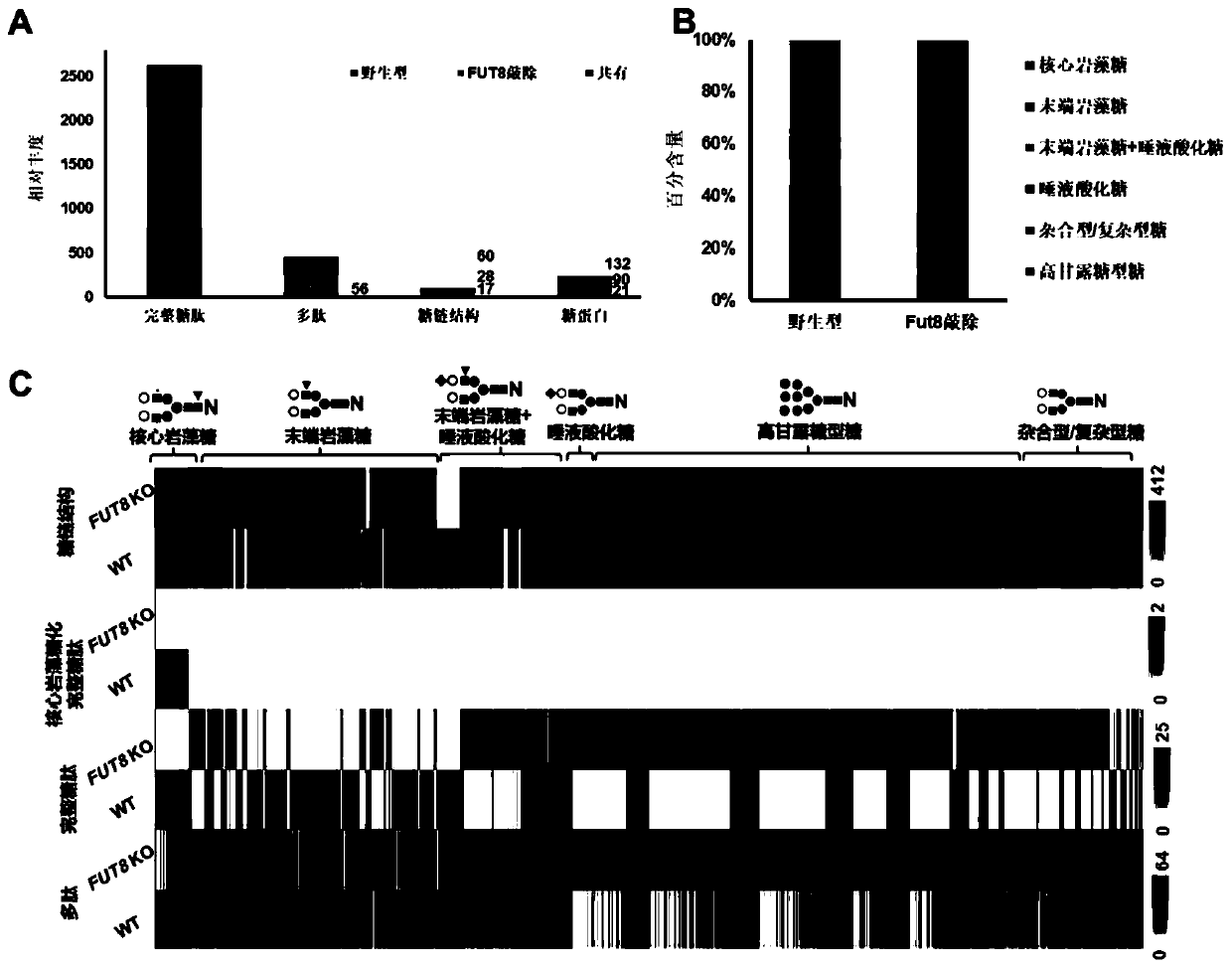
[0056] Analysis of WT and Fut8 knockout (Fut8 - / -) Complete glycopeptides of glycoengineered CHO cells:
[0057] We applied the one-step enrichment method of Example 1 to the analysis of wild-type (WT) and glycoengineered Chinese hamster ovary (CHO) cells, and evaluated the N-linked glycosylation and even Changes in glycoprotein expression. By knocking out the Fut8 gene in CHO cells (Fut8 - / - ) can generate core fucosylation-deficient cell lines. Fut8 (α1,6-fucosyltransferase) catalyzes the transfer of a fucose residue to position 6 of the innermost GlcNAc residue of an N-linked oligosaccharide of a glycoprotein (ie, core fucosylation). Based on the glycopeptides obtained by the one-step enrichment method, after LC-MS / MS analysis, the screening criteria of the database searched by GPQuest are as follows: (1) glycopeptide error rate (FDR) is less than 1%, (2) each peptide requires PSM ≥2, (3) all PSMs should annotate at least one N-linked glycan, (4) the core Fuc fragment i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com