Preparation method of high-purity boehmite and high-purity aluminum oxide
A technology of boehmite and boehmite, which is applied in the field of preparation of high-purity boehmite and high-purity alumina, can solve the problems of high content, difficult to continue to improve the purity, and affect the application, and achieve high purity, The effect of low price and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The first aspect of the present invention provides a method for preparing high-purity boehmite, the steps comprising:
[0023] S1. Acid-dissolving: acid-dissolving industrial aluminum hydroxide to obtain aluminum salt solution;
[0024] S2, crystallization and purification: crystallization and purification of the aluminum salt solution obtained in step S1 to obtain high-purity aluminum salt;
[0025] S3. Pre-precipitation and impurity removal: add a pre-precipitant to the aqueous solution of the high-purity aluminum salt to form a colloidal precipitation, and separate the solid and liquid to obtain a high-purity aluminum salt solution; the pre-precipitant includes one of ammonia water, ammonium carbonate or ammonium bicarbonate species or several;
[0026] S4. Chemical homogeneous precipitation: react the high-purity aluminum salt solution obtained in step S3 with a precipitant, control the reaction temperature to 85-99° C., and the pH of the reaction end point to 5.5-...
Embodiment 1
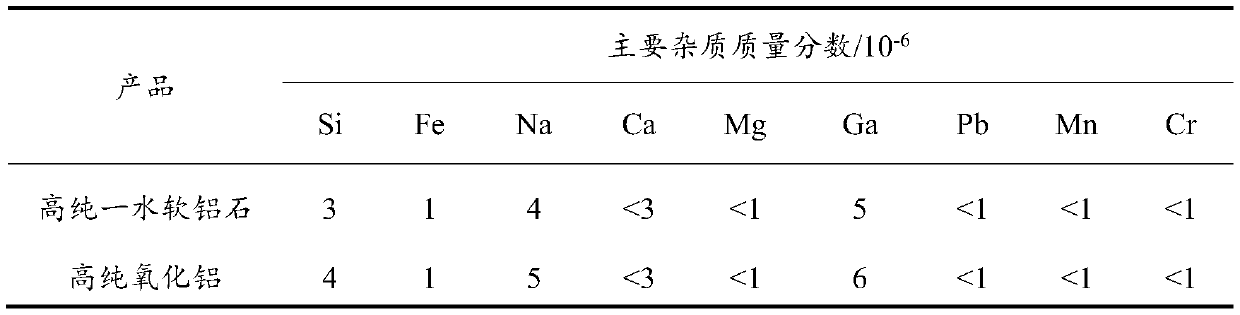
[0044]In this example, industrial aluminum hydroxide is dissolved in sulfuric acid to obtain an aluminum sulfate solution, and high-purity aluminum sulfate is obtained by two crystallization purifications. A certain amount of ammonium carbonate is added to form a highly active flocculent colloid for impurity removal, and high-purity sulfuric acid is obtained after solid-liquid separation. aluminum solution. Precipitate reaction between urea and high-purity aluminum sulfate solution to obtain amorphous alumina hydrate, obtain high-purity boehmite through hydrothermal treatment, and obtain high-purity alumina after roasting. Specific steps are as follows:
[0045] (1) Acid-soluble industrial aluminum hydrogen: configure sulfuric acid with a concentration of 30%, heat and dissolve industrial aluminum hydroxide under stirring conditions, and obtain aluminum sulfate solution after dilution and filtration. In order to ensure the complete consumption of sulfuric acid, control the exc...
Embodiment 2
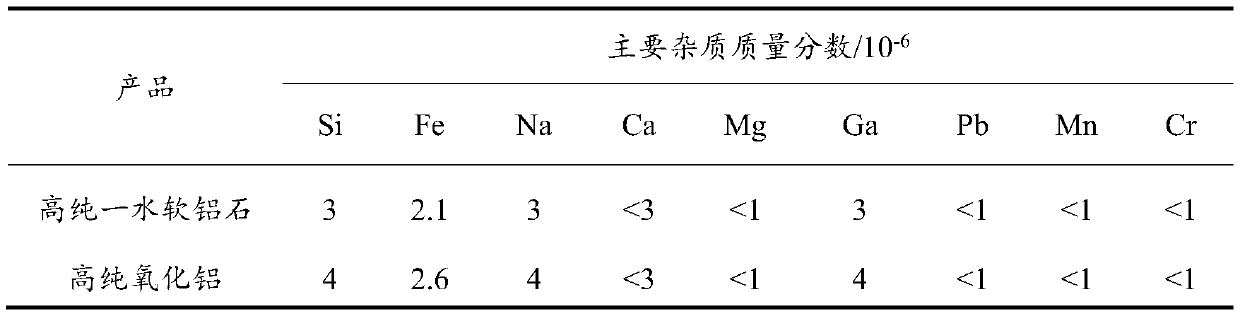
[0055] In this example, industrial aluminum hydroxide is dissolved in nitric acid to obtain aluminum nitrate solution, and high-purity aluminum nitrate is obtained by two crystallization purifications, and a certain amount of ammonia water is added to form highly active floc colloid for impurity removal, and high-purity aluminum nitrate is obtained after solid-liquid separation solution. Precipitation reaction between urea and high-purity aluminum nitrate solution to obtain amorphous alumina hydrate, high-purity boehmite after hydrothermal treatment, and high-purity alumina after roasting. Specific steps are as follows:
[0056] (1) Acid-soluble industrial aluminum hydroxide: prepare nitric acid with a concentration of 30% to heat and dissolve industrial aluminum hydroxide, and obtain an aluminum nitrate solution after dilution and filtration.
[0057] (2) Twice crystallization and purification: cooling the aluminum nitrate solution to 20°C, filtering to obtain primary crysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com