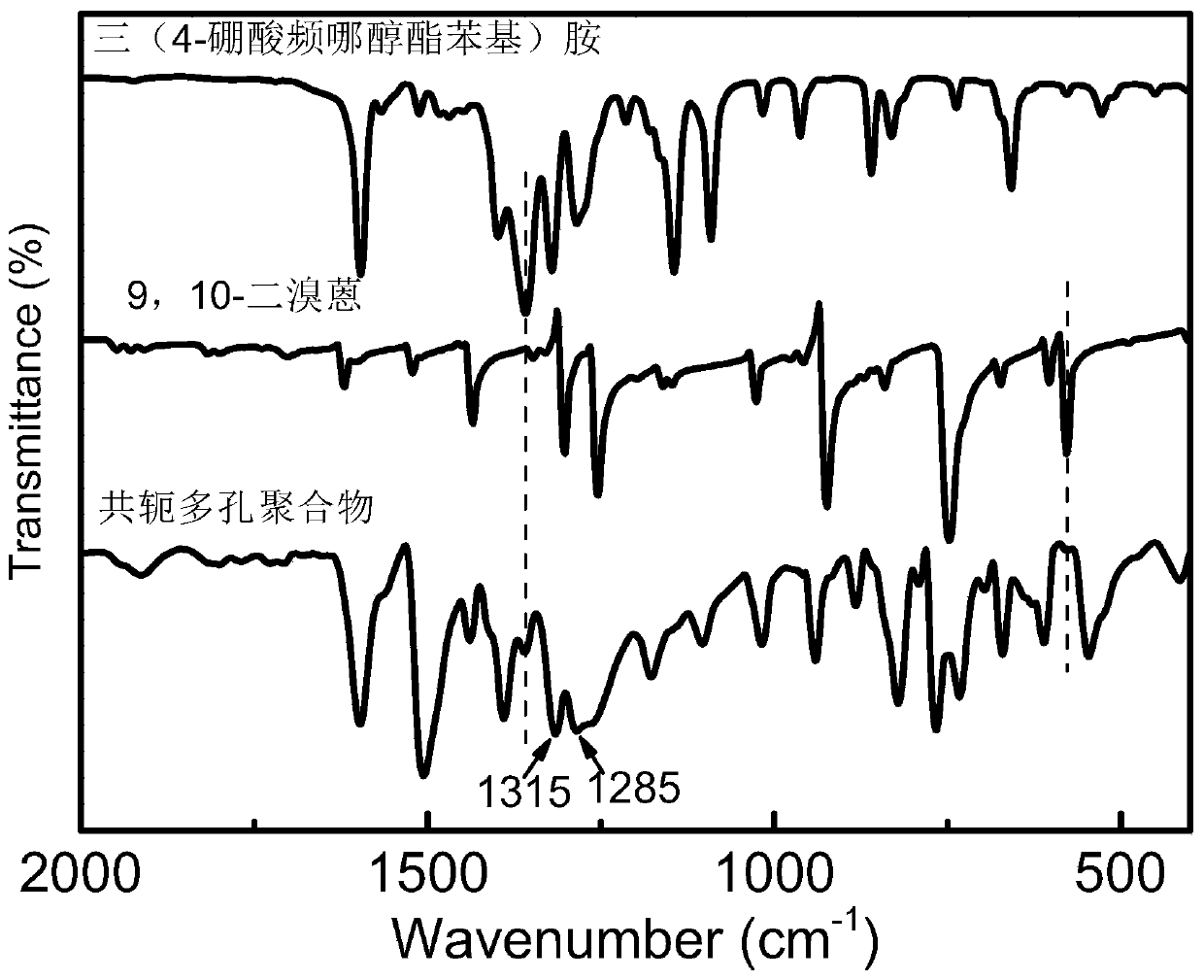
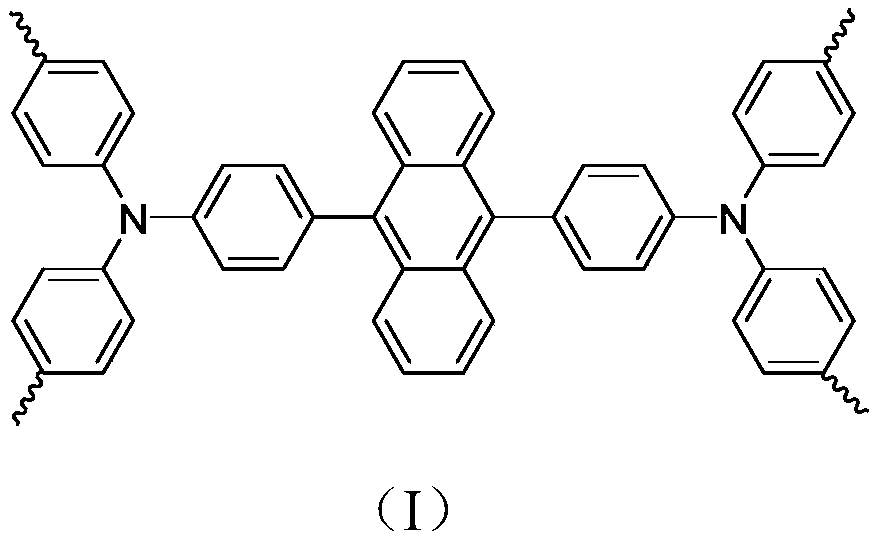
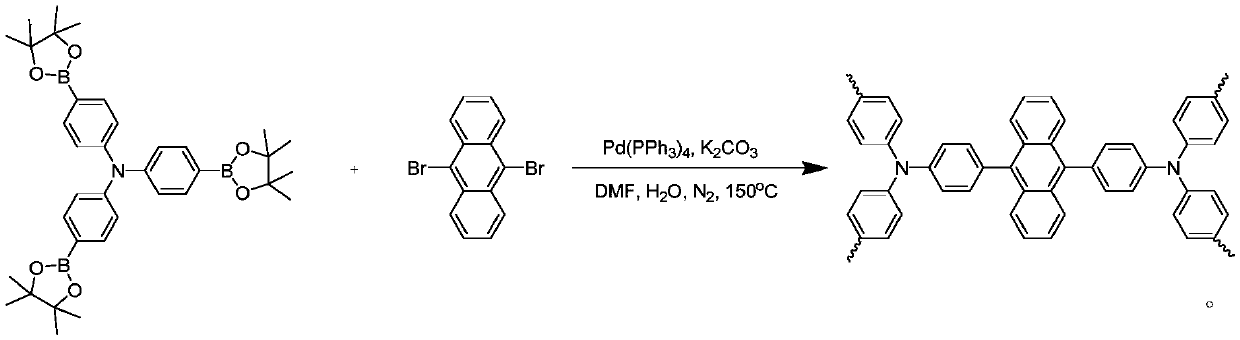
Conjugated porous polymer, preparation method thereof, and application thereof in imine preparation through photo-catalyzed oxidation of primary amine
A porous polymer and conjugated technology, applied in the direction of imino compound preparation, catalytic reaction, organic compound/hydride/coordination complex catalyst, etc., can solve the problems of poor catalyst stability, difficult catalyst recovery, high cost, etc. Achieve mild reaction conditions, high chemical stability and thermal stability, easy separation and recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Using 0.5mmol benzylamine as a raw material, add 5mL acetonitrile and 5mL water as a mixed solvent, and add oxygen as an oxidant and bubble for 10min. 20 mg of a D-π-D type conjugated porous polymer was added as a catalyst. The catalytic reaction was carried out at room temperature and under the irradiation of white light LED for 24 h. The catalyst was removed by filtration, extracted with dichloromethane and concentrated by rotary evaporation to obtain the corresponding product imine.
Embodiment 2
[0032] Using 0.5 mmol of p-bromobenzylamine as a raw material, add 5 mL of acetonitrile and 5 mL of water as a mixed solvent, and add oxygen as an oxidant and bubble for 10 min. 20 mg of a D-π-D type conjugated porous polymer was added as a catalyst. The catalytic reaction was carried out at room temperature and under the irradiation of white light LED for 24 h. The catalyst was removed by filtration, extracted with dichloromethane and concentrated by rotary evaporation to obtain the corresponding product imine.
Embodiment 3
[0034] Using 0.5 mmol of 3-aminomethylpyridine as a raw material, add 5 mL of acetonitrile and 5 mL of water as a mixed solvent, and add oxygen as an oxidant and bubble for 10 min. 20 mg of a D-π-D type conjugated porous polymer was added as a catalyst. The catalytic reaction was carried out at room temperature and under the irradiation of white light LED for 24 h. The catalyst was removed by filtration, extracted with dichloromethane and concentrated by rotary evaporation to obtain the corresponding product imine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com