Microorganism and use thereof
A technology of microorganisms and plasmids, applied in the field of bioengineering, can solve the problems of residual chemical reagents, low farnesene content, and inability to apply, and achieve the effects of overcoming difficulties in genetic manipulation, improving yield and efficiency, and short production cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The recombinant escherichia coli of the optimal collocation of embodiment 1MVA and MEP approach
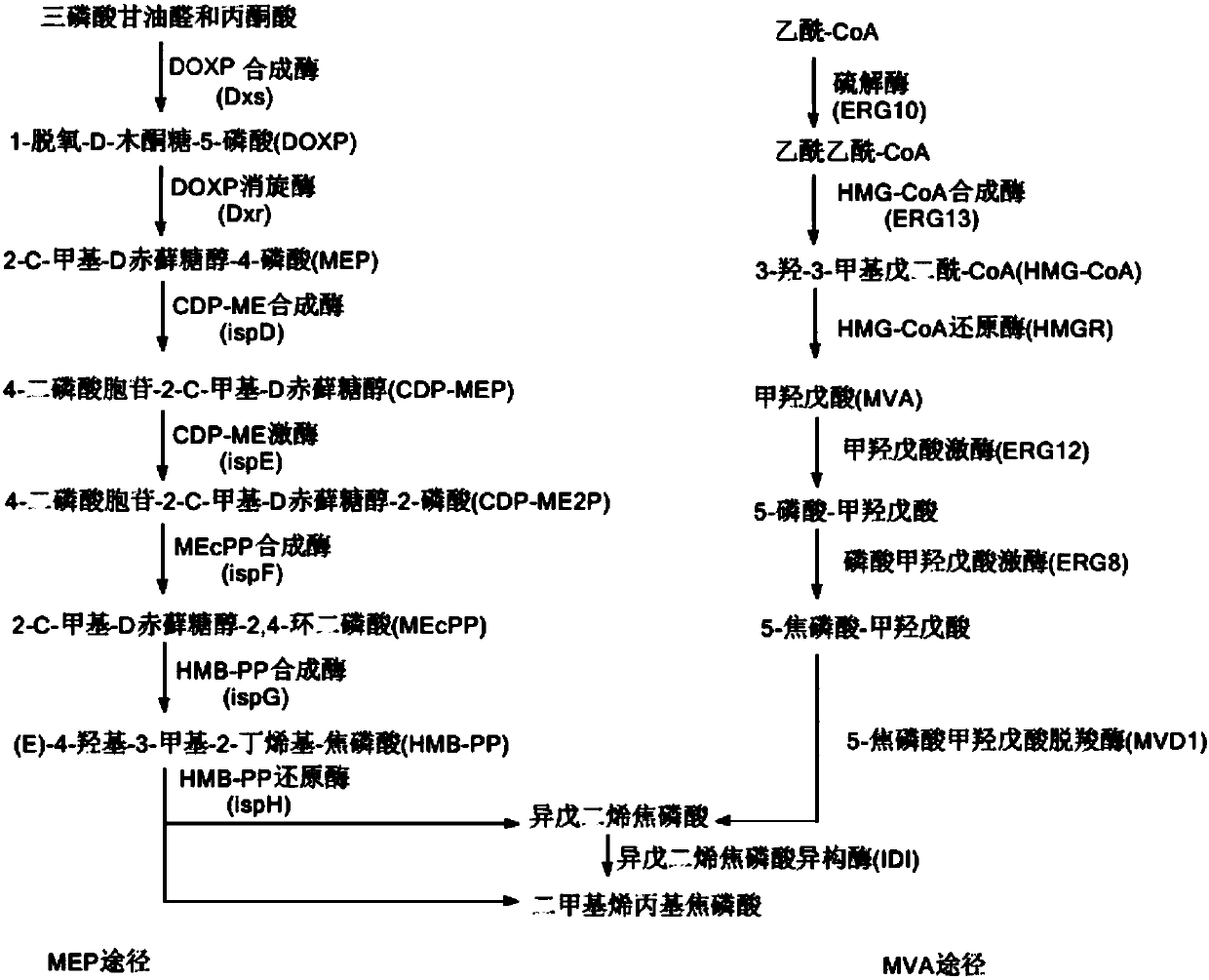
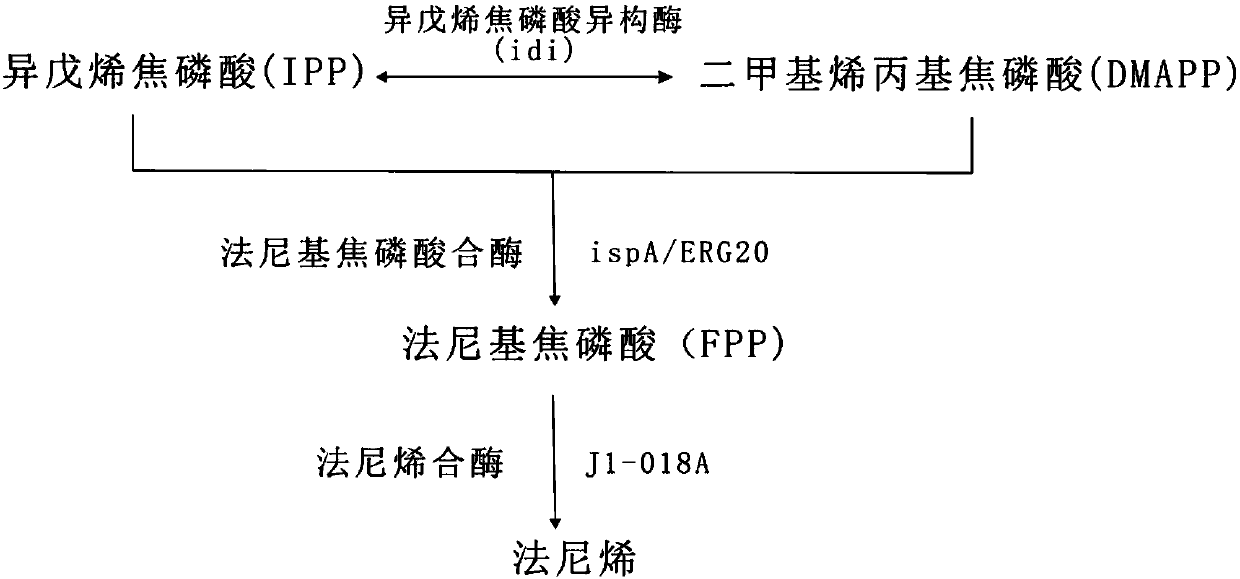
[0018] Isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) are two general substrates for isoprenoid biosynthesis. Two isoprenoid pathways, the methylerythritol phosphate (MEP) pathway and the mevalonate (MVA) pathway, are responsible for IPP and DMAPP synthesis in nature. The MEP pathway is more efficient than the MVA pathway in converting carbon sources (eg, glucose, glycerol) into isoprenoids. However, energy and reducing power will be consumed in this process, so additional energy molecule ATP and reducing equivalent NADPH provided from other ways are needed. At the same time, the MVA pathway, which overproduces isoprenoids, produces excess reducing equivalent NADH. How can the MVA pathway be used to provide the reducing equivalents required for the MEP pathway? By changing the flux of the MEP pathway and the MVA pathway, a more balanced metabolic pat...
Embodiment 2
[0025] The optimal matching recombinant yeast of embodiment 2MVA and MEP approach
[0026] At the same time, the inventor also constructed recombinant Saccharomyces cerevisiae based on this theoretical content. Plasmid pJ1-018-11 contains ERG10, ERG12, and tHMG1 in the MVA pathway of Saccharomyces cerevisiae. These genes were obtained by PCR, and the control gene expression was pGAL1 and pGAL7 respectively. , pGAL10 promoter. These fragments were connected sequentially by designing primers to form a 50bp overlapping sequence between two adjacent genes, with 1.5kb sequences homologous to the integration site on both sides of the target fragment, and these fragments were cloned in the DNA assembly method. The pRS423 vector contained a NotI restriction site between the fragment and the vector, and the positive clone was sequenced and named pJ1-018-11.
[0027] Plasmid pJ1-018-12 contains Erg13, ERG8, and MVD1 in the MVA pathway of Saccharomyces cerevisiae. These genes are obtain...
Embodiment 3
[0034] The optimal matching recombinant Streptomyces of embodiment 3MVA and MEP approach
[0035] According to the above conclusions, when the flux ratio of the MEP pathway to the MVA pathway is 4:3, farnesene can be produced more efficiently, so the inventors made the related proteins ERG10, ERG12, tHMG1, Erg13, ERG8 in the Saccharomyces cerevisiae MVA pathway , MVD1 and ERG20 were cloned on pIB139 according to the Gibson method, and the positive clone was sequenced and named pJ1-018-26. The plasmid controls gene expression by a low-strength ermE promoter, contains attP sites and Int integrase, and is resistant to abramycin.
[0036]Using PCR-targetting (Gust. B., 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of thesesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A. 100 (4): 1541-1546.) Plasmid pSET152 (Qian Liu.,2016.Development of Streptomyces sp.FR-008as anemerging chassis.Synth Syst Biotechnol.1(3):207-214.) T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com