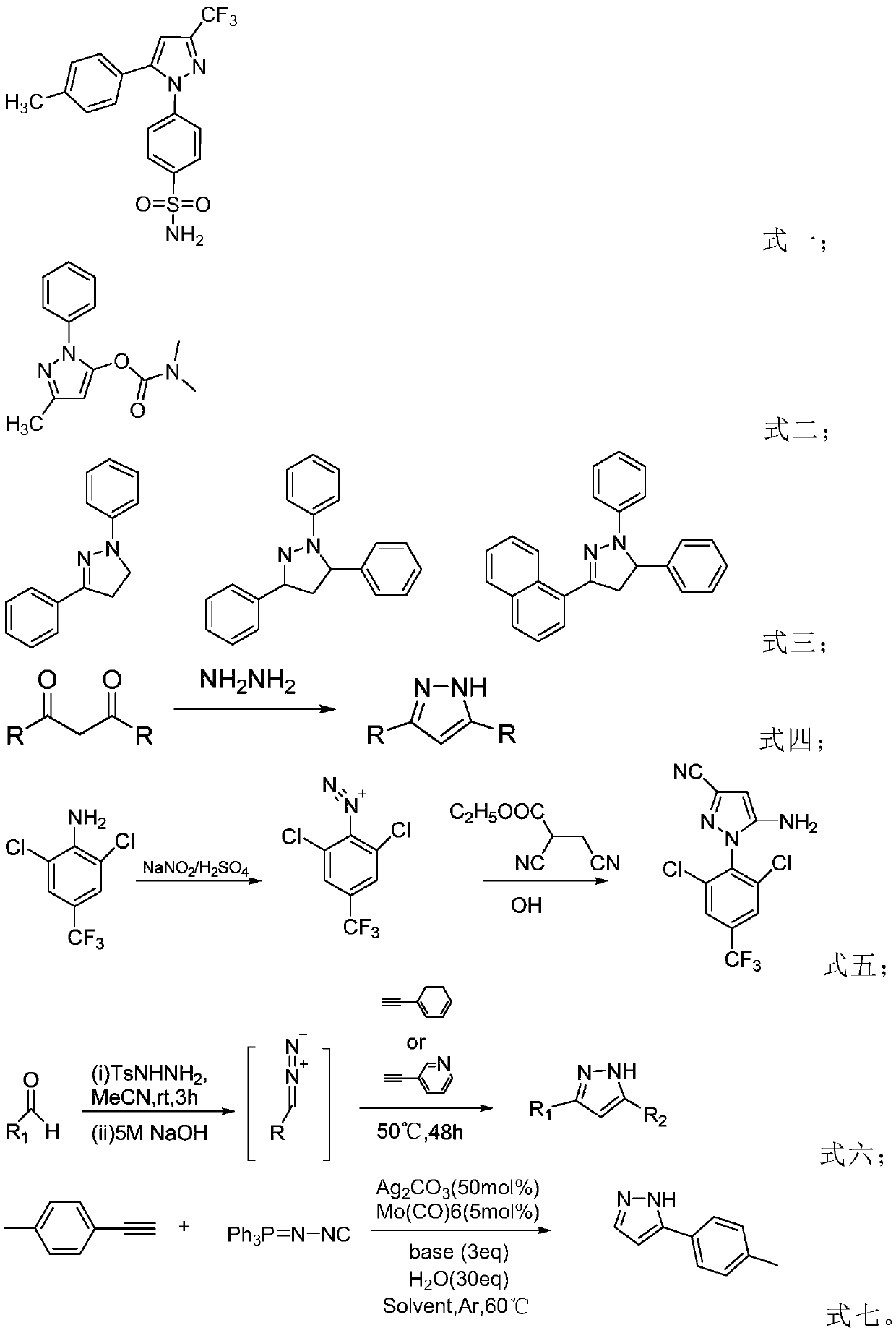
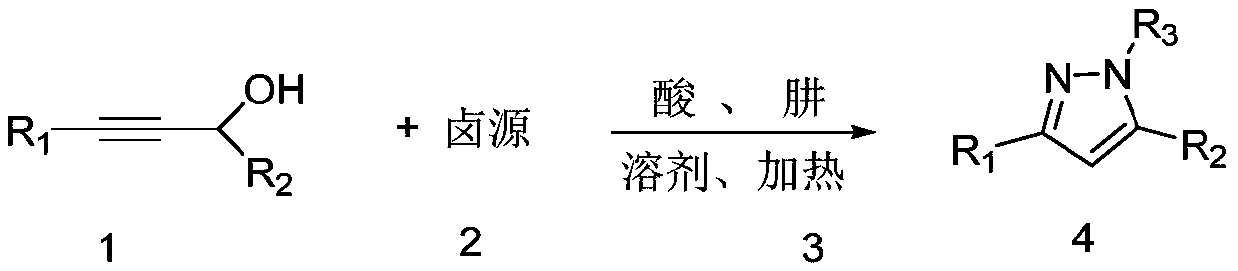
Preparation method of pyrazole derivative
A technology of pyrazole derivatives and derivatives, applied in organic chemistry and other fields, can solve the problems of target product yield of 35-68%, limitation of substrate universality, yield to be improved, etc., and reach the scope of application of substrates Wide range, reduced environmental pressure, and high atomic utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention is a kind of preparation method of pyrazole derivative, comprises the following steps:
[0032] (1) Add a propargyl alcohol derivative, a halogen source, an acid and a solvent into a sealed tube, mix to obtain a reaction solution, heat, and react at 40-120° C., and the complete disappearance of the propargyl alcohol derivative is monitored by TLC;
[0033] (2) Add hydrazine compounds to the reaction solution obtained in step (1), add saturated brine to quench after reacting for 0.5h-5h, extract the organic phase with ethyl acetate, dry over anhydrous sodium sulfate, concentrate to obtain pyrazole derivatives The product purity can be improved by column chromatography; wherein the molar ratio of propargyl alcohol derivative, halogen source, hydrazine compound and acid is 1: (1.0~2.0): (0.5~3.0): (0.01~0.5); The feed ratio of propargyl alcohol derivative and solvent is 1:5~1:20mmol / mL;
[0034] The preparation method of the present invention uses pr...
Embodiment 1
[0052] A kind of preparation method of pyrazole derivative, pyrazole derivative is specifically 3-phenyl-1H-pyrazole, and its structural formula is as follows:
[0053]
[0054] The preparation process is as follows: add propargyl alcohol derivatives, halogen source and acid in a sealed tube, react under reflux at 101°C, add hydrazine after the complete disappearance of propargyl alcohol derivatives as monitored by TLC, add saturated Quench with saline, extract the organic phase with ethyl acetate, dry over anhydrous sodium sulfate, and concentrate to obtain pyrazole derivatives. The purity of the product can be improved by column chromatography, and the calculated yield is 91.0%. Among them, propargyl alcohol, halogen source, hydrazine, solvent and acid are added as 3-phenylprop-2-yn-1-ol (2mmol), NBS (2mmol), hydrazine hydrate (2.1mmol), dioxane (10 mL) and bismuth triflate (0.1 mmol).
[0055] The prepared product was characterized, and its NMR characterization data wer...
Embodiment 2
[0057] A kind of preparation method of pyrazole derivative, pyrazole derivative is specifically 3-(4-bromophenyl)-1H-pyrazole, and its structural formula is as follows:
[0058]
[0059] The preparation process is as follows: add propargyl alcohol derivatives, halogen source and acid in a sealed tube, react under reflux at 101°C, add hydrazine after the complete disappearance of propargyl alcohol derivatives as monitored by TLC, add saturated Quench with brine, extract the organic phase with ethyl acetate, dry over anhydrous sodium sulfate, and concentrate to obtain pyrazole derivatives. The purity of the product can be improved by column chromatography, and the calculated yield is 89%. Among them, propargyl alcohol, halogen source, hydrazine, solvent and acid are added as 1,3-diphenylprop-2-yn-1-ol (2mmol), NIS (2.2mmol), hydrazine hydrate (3.2mmol) , dioxane (20 mL) and copper triflate (0.3 mmol).
[0060] The prepared product was characterized, and its nuclear magnetic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com