Synthesis method of rosuvastatin tert-butyl ester
A technology for the synthesis of rosuvastatin tert-butyl ester and its synthesis method, which is applied in the field of synthesis of rosuvastatin tert-butyl ester, can solve the problems of difficult acquisition of raw materials, difficulty in realizing industrialized production, complicated routes, etc., and achieve broad prospects and industrial applications Value, reduction of waste pollution, convenient and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] A synthetic method of rosuvastatin tert-butyl ester, comprising the steps of:
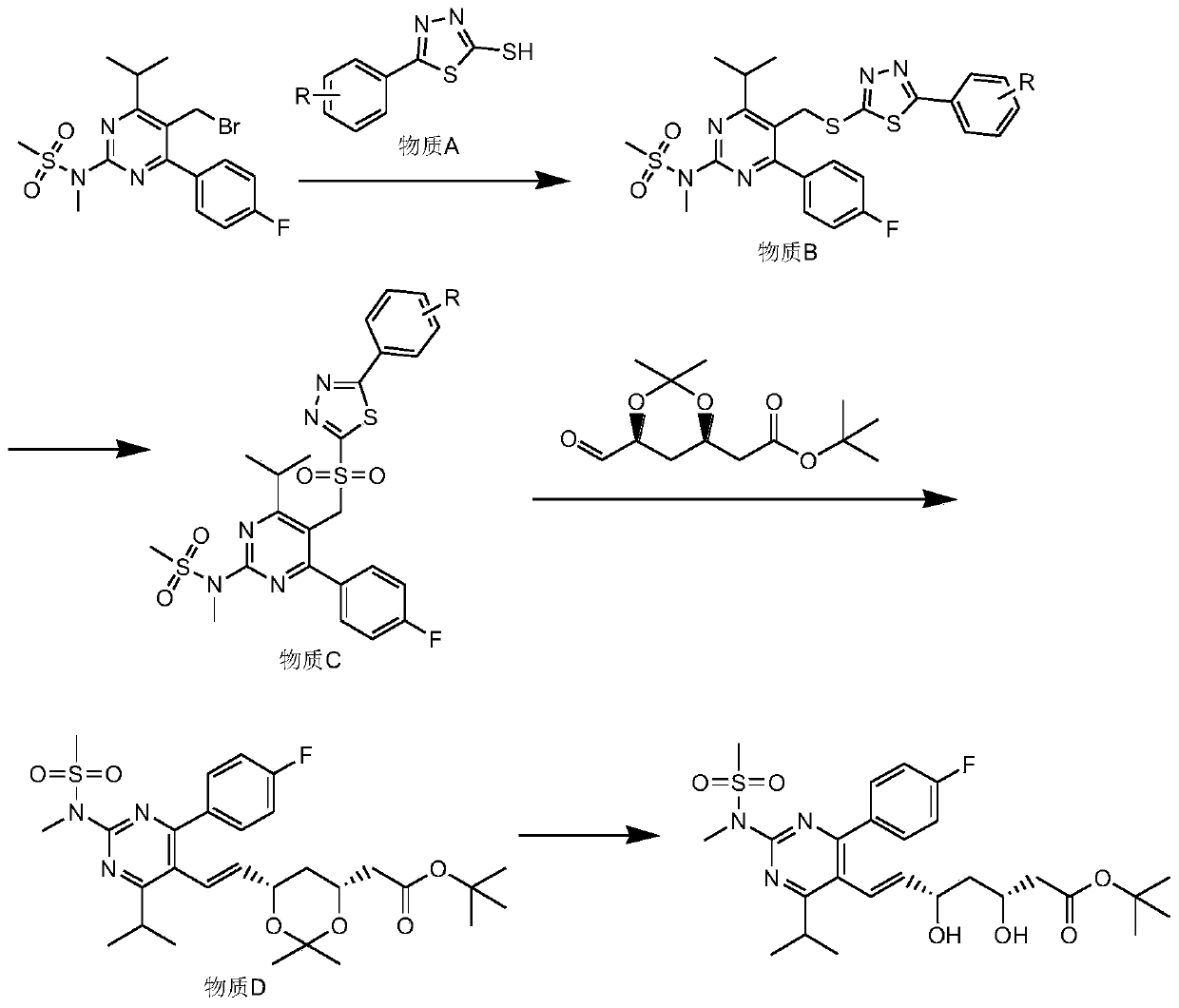
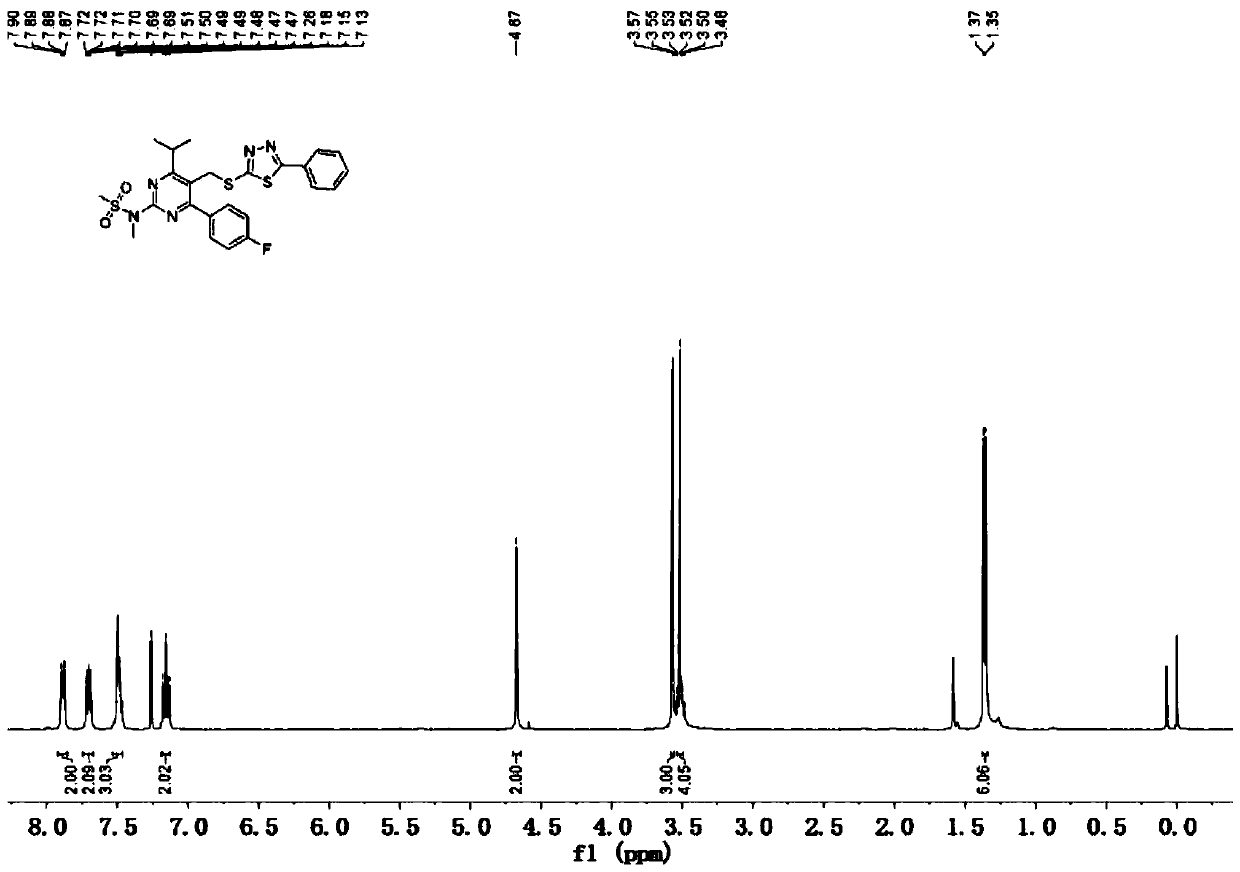
[0062]Add 38g (0.36mol) of sodium carbonate, 150mL of water, and 250mL of acetone into a 1000mL four-necked round-bottom flask in sequence, then add 5-bromomethyl-4-(4-fluorophenyl)-6-isopropyl Base-2-[methyl(methylsulfonyl)amino]pyrimidine 100g (0.24mol), 2-mercapto-5-phenyl-1.3.4-thiadiazole 56g (0.288mol), slowly warming up to 50°C, stirring React for 4 hours, take samples for TLC (eluent: petroleum ether: ethyl acetate = 1:1v / v) to detect complete conversion of raw materials, cool down to 0°C, continue to stir for 1 hour, filter, 500mL water beating to obtain the wet product of substance B;
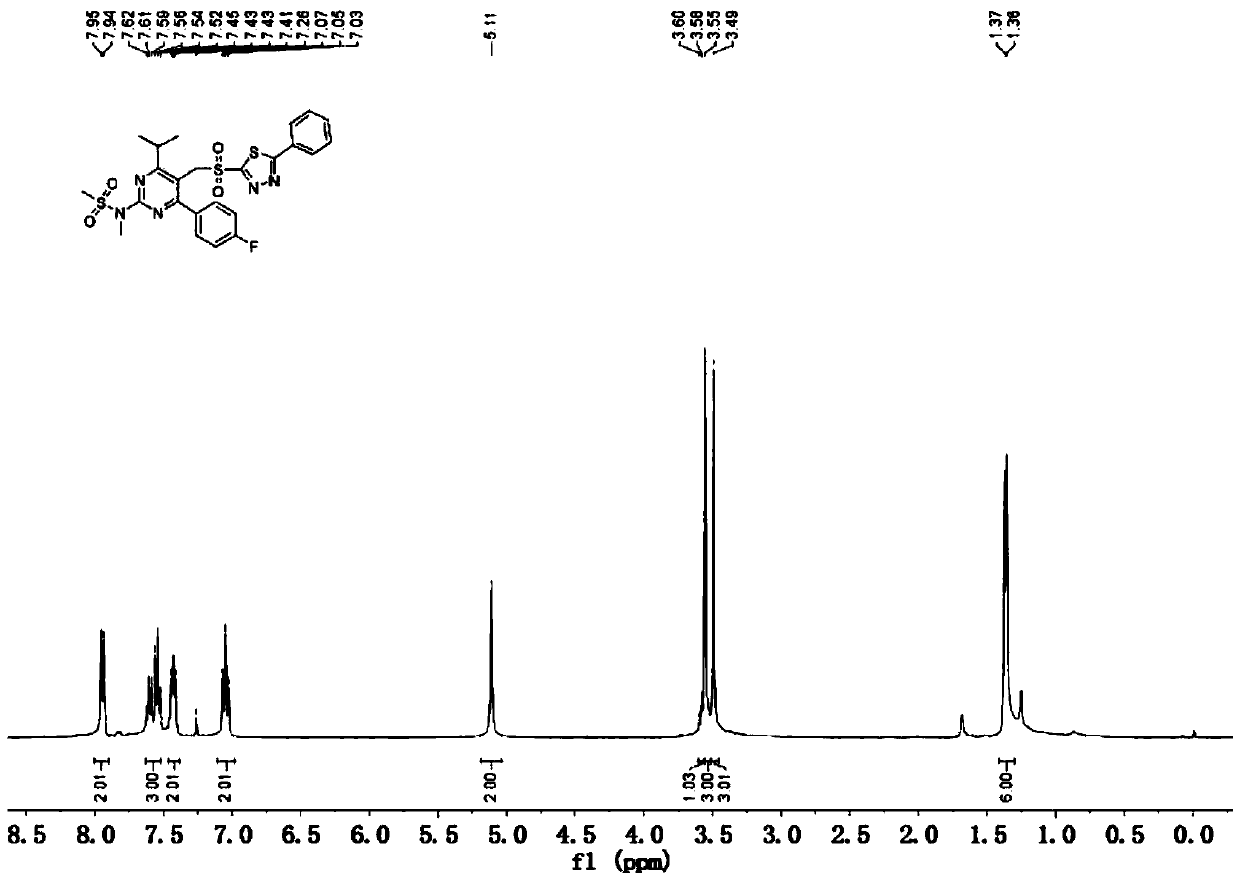
[0063] In a 1000mL four-neck round bottom flask, add the wet product of substance B (theoretical weight 127.3g, 0.24mol), ethanol 635mL, ammonium molybdate tetrahydrate 14.9g (0.012mol), and slowly add 30% hydrogen peroxide 163.5 g (that is, the hydrogen peroxide is 1.44mol), the dropwise addition ...
Embodiment 2
[0068] Replace 2-mercapto-5-phenyl-1.3.4-thiadiazole with 2-mercapto-5-(2-methylphenyl)-1.3.4-thiadiazole, sodium carbonate with potassium hydroxide, 2- The molar ratio of mercapto-5-(2-methylphenyl)-1.3.4-thiadiazole to potassium hydroxide is 1:0.5;
[0069] Replace molybdic acid tetrahydrate with sodium tungstate, the molar ratio of substance B to sodium tungstate is 1:0.5, and the molar ratio of substance B to hydrogen peroxide is 1:1;
[0070] Sodium tert-butoxide was replaced by sodium methoxide, and the molar ratio of substance C to sodium methoxide was 1:0.5; the others were the same as in Example 1.
Embodiment 3
[0072] Replace 2-mercapto-5-phenyl-1.3.4-thiadiazole with 2-mercapto-5-(2-bromophenyl)-1.3.4-thiadiazole, replace sodium carbonate with lithium carbonate, 2- The molar ratio of mercapto-5-(2-bromophenyl)-1.3.4-thiadiazole to lithium carbonate is 1:5;
[0073] The molar ratio of substance B to molybdic acid tetrahydrate is 1:0.01, and the molar ratio of substance B to hydrogen peroxide is 1:20;
[0074] Sodium tert-butoxide was replaced by lithium bis(trimethylsilyl)amide, and the molar ratio of substance C to lithium bis(trimethylsilyl)amide was 1:10; the others were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com