Human-derived probiotic agent and application thereof in assisting in reducing blood sugar
A probiotic and human-derived technology, applied in the field of microorganisms, can solve problems such as high cost and long drug research cycle, and achieve the effects of slowing down decomposition, assisting in lowering blood sugar, and relieving symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Strain Isolation and Identification
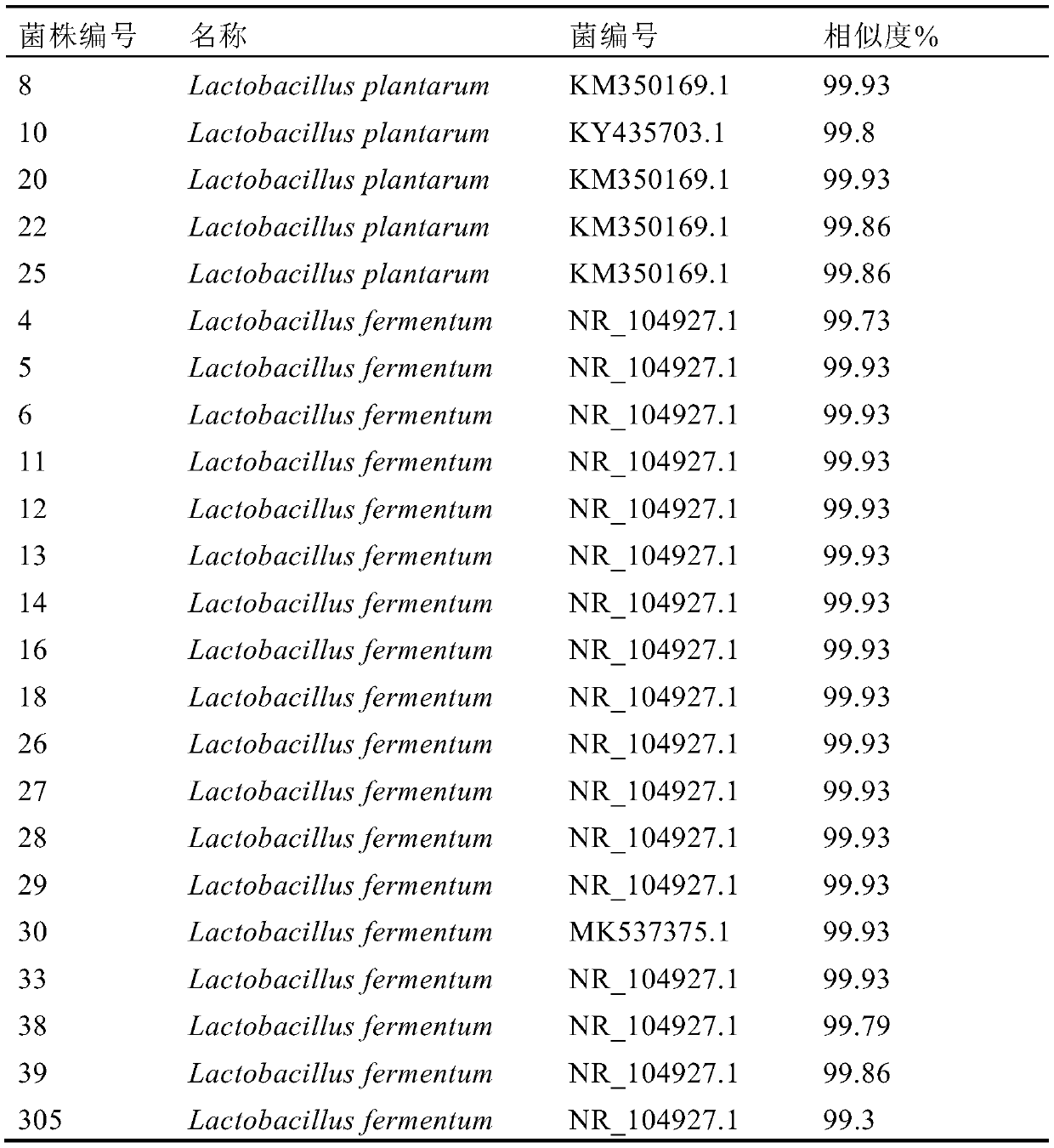
[0049] Take 10g of feces from healthy people and put them into 100mL MRS broth culture medium for 24h, then carry out gradient dilution, select the appropriate gradient (10 -6 、10 -7 、10 -8 ) onto the MRS solid plate. After static culture at 37°C for 24 hours, colonies were selected according to the size, shape and color of the colonies, stored on a slant and numbered, and all screened strains were identified by 16S rDNA molecules, and 5 strains of Lactobacillus plantarum and 18 strains of Lactobacillus fermentum were obtained. As shown in Table 1.
[0050] Table 1 16S rDNA molecular identification of isolated strains
[0051]
Embodiment 2
[0053] 1. Determination of the number of viable bacteria
[0054] The above-mentioned 5 strains of Lactobacillus plantarum and 18 strains of Lactobacillus fermentum were respectively picked from their respective preserved slant surfaces and cultured for 12 hours at 37°C in MRS broth medium, and the activated The strain culture solution was inoculated into MRS broth medium, and cultured at 37°C for 12 hours. Take 0.9mL bacterial solution for 10-fold serial dilution. Choose a suitable gradient (10 -6 、10 -7 、10 -8 ) for coating. Take 0.1mL bacterial solution from each gradient and place it on the MRS solid plate, spread it evenly with a spreader, let it stand for 1 hour, then place it upside down in a constant temperature incubator at 37°C for 24 hours, and count the colonies.
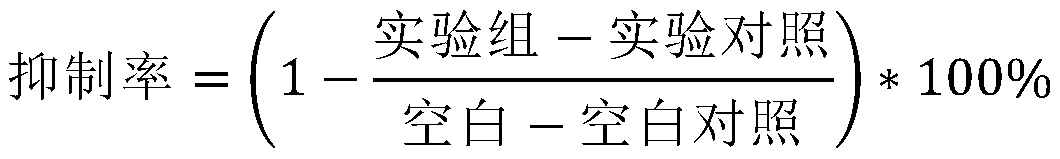
[0055] The determination of the number of viable bacteria by calculating the concentration of bacteria after fermentation is to understand the growth of bacteria and provide data support for subsequ...
Embodiment 3
[0070] 1 Simulated gastrointestinal digestion
[0071] In order to verify the viability of lactic acid bacteria in the gastrointestinal tract, 4 strains of Lactobacillus plantarum and 7 strains of Lactobacillus fermentum with higher comprehensive evaluation of the activity inhibition rate of α-amylase and α-glucosidase in Table 2 were selected to simulate the gastrointestinal tract Digestion experiment.
[0072] According to De-QuanZhu et al. (Zhu D Q, Liu F, Sun Y, et al. Genome-Wide Identification of Small RNAs in Bifidobacterium animalis subsp. lactis KLDS2.0603 and Their Regulation Role in the Adaption to GastrointestinalEnvironment[J].Plos One, 2015,10(2):e0117373.) to make artificial saliva, gastric juice and intestinal juice. Take a ring of bacteria on the slant and inoculate into 10mL MRS broth medium, inoculate it in a constant temperature incubator at 37°C for 12 hours, then inoculate it into 30mL liquid medium with 5% inoculum amount, and then culture it in a const...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com