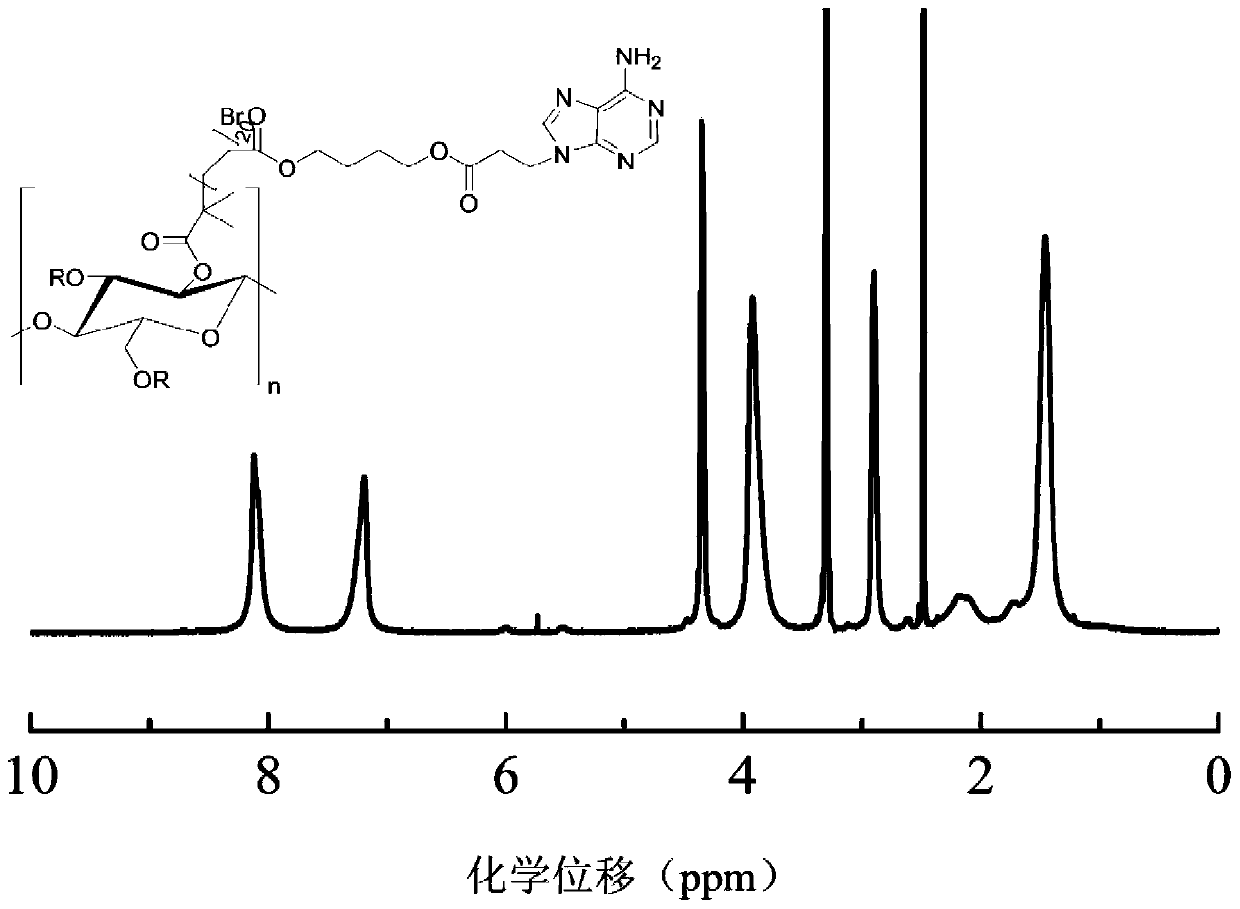
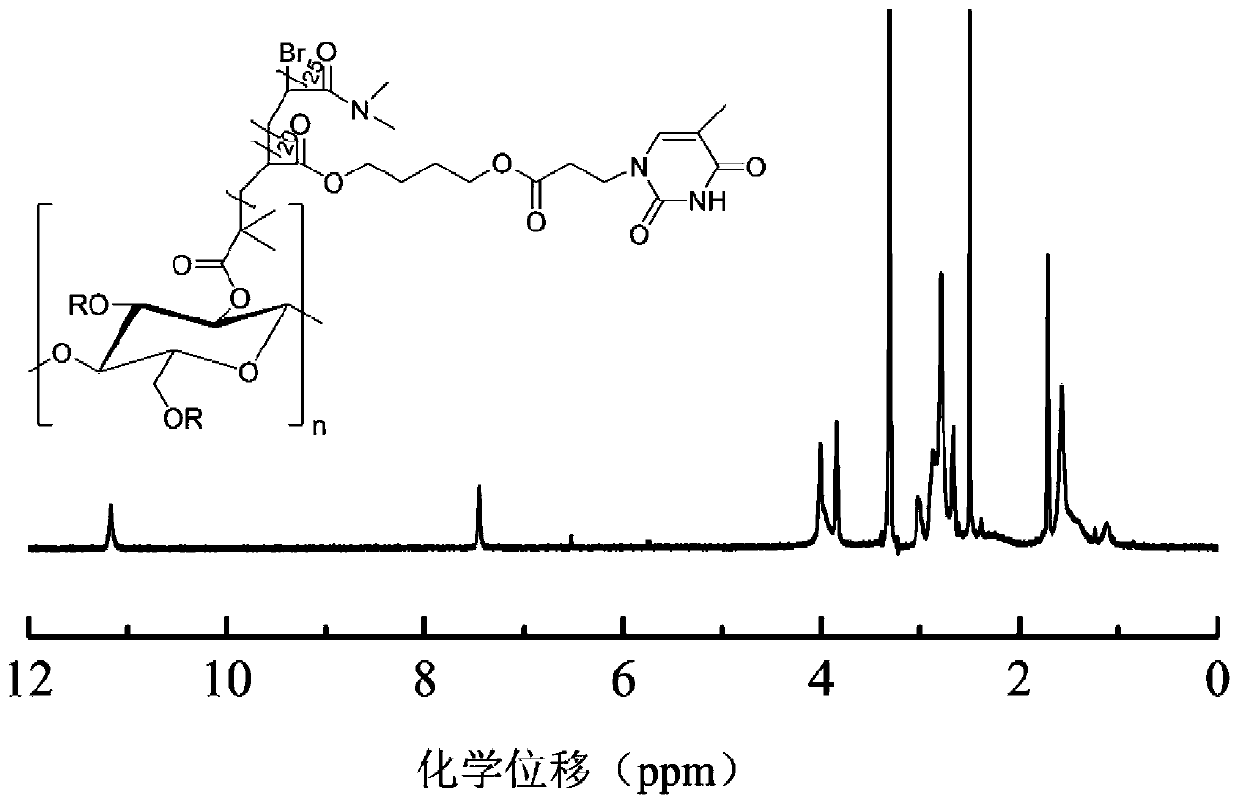
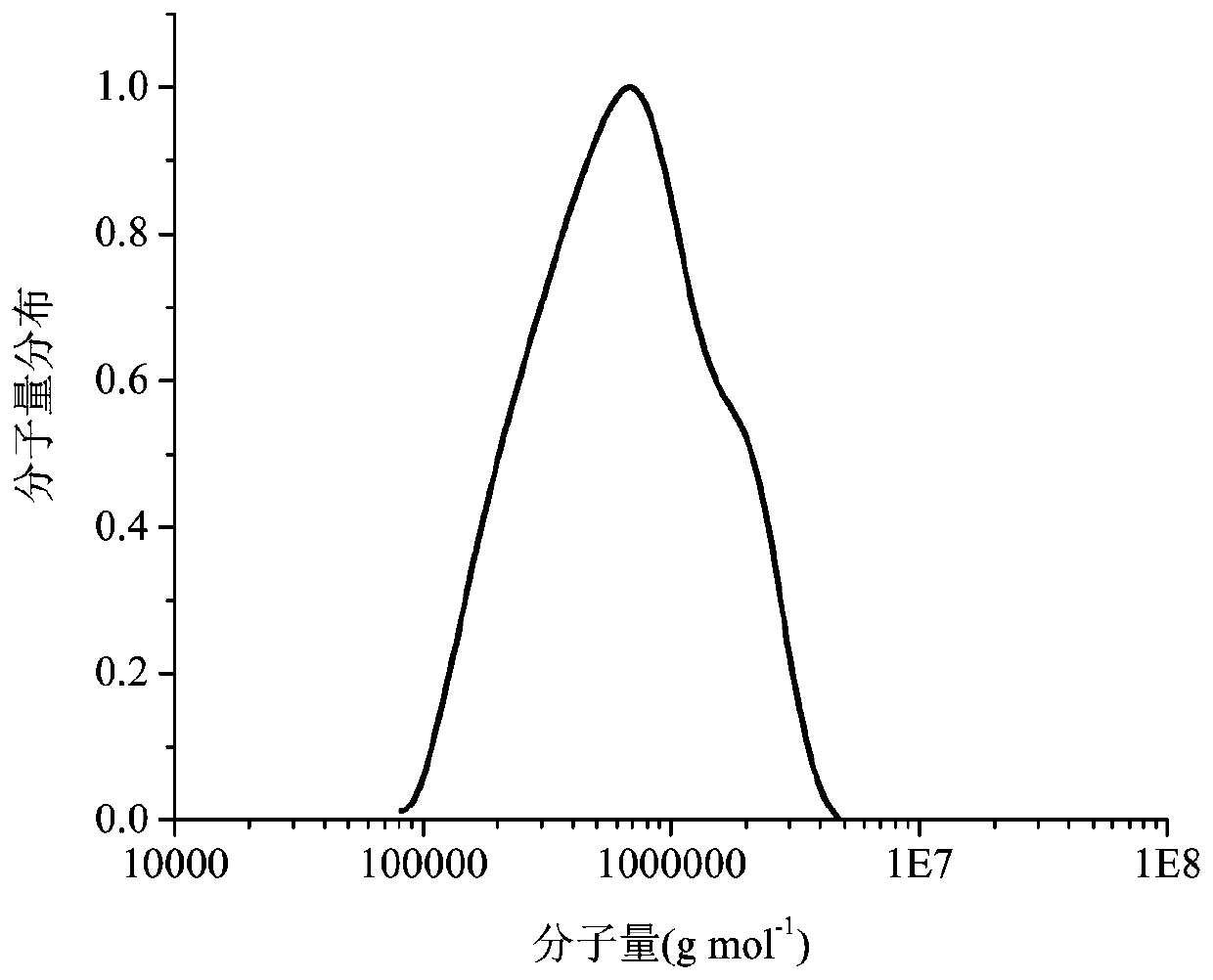
Amphipathic nucleobase functionalized cellulose polymer, micelle and preparation method thereof
A technology of cellulose polymer and nucleobase function, which is applied in the field of polymer material preparation, can solve the problems of high synthesis cost, wide application and limitation, and achieve the effect of high-value utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Preparation method of amphiphilic nucleobase functionalized cellulose polymer micelles
[0123] (1) Preparation of cellulose macromolecular initiator
[0124] (1) Weigh 25g of 1-allyl-3-methylimidazolium chloride salt and 1g of microcrystalline cellulose, respectively, and dry them under vacuum at 80°C for 24h;
[0125] (2) Add microcrystalline cellulose to 1-allyl-3-methylimidazolium chloride salt, pump with water at 80°C for 2 hours, and then pump with oil for 1 hour;
[0126] (3) Slowly add 16mL of N,N-dimethylformamide to the mixture in step (2), react for 10min, and take an ice-water bath;
[0127] (4) Add 5 mL of 2-bromoisobutyryl bromide to the mixture of step (3), react at room temperature for 18 hours, purify the reaction product with methanol, precipitate the product in methanol, and dry to obtain a cellulose macromolecule Initiator.
[0128] (2) Preparation method of adenine acrylate monomer
[0129] (1) Weigh 13.5g of adenine, 0.811g of 2,6-di-tert-butyl...
Embodiment 2
[0142] Preparation method of amphiphilic nucleobase functionalized cellulose polymer micelles
[0143] (1) Preparation of cellulose macromolecular initiator
[0144] (1) Weigh 48g of 1-allyl-3-methylimidazolium chloride salt and 2g of microcrystalline cellulose, respectively, and vacuum-dry them at 80°C for 24h;
[0145] (2) Add microcrystalline cellulose to 1-allyl-3-methylimidazolium chloride salt, pump with water at 80°C for 2 hours, and then pump with oil for 1 hour;
[0146] (3) Add 30mL of N,N-dimethylformamide to the mixture in step (2), react for 10min, and take an ice-water bath;
[0147] (4) Add 10mL of 2-bromoisobutyryl bromide to the mixture of step (3), react at room temperature for 18h, purify the reaction product with methanol, precipitate the reaction product in methanol, and dry to obtain cellulose large molecular initiator.
[0148] (2) Preparation method of thymine acrylate monomer
[0149] Weigh 12.6g of thymine, 0.811g of 2,6-di-tert-butyl-4-methylphen...
Embodiment 3
[0164] Preparation method of amphiphilic nucleobase functionalized cellulose polymer micelles
[0165] (1) Preparation of cellulose macromolecular initiator
[0166] (1) Weigh 10 g of 1-allyl-3-methylimidazolium chloride salt and 0.5 g of microcrystalline cellulose, respectively, and vacuum-dry them at 80° C. for 24 hours;
[0167] (2) Add microcrystalline cellulose to 1-allyl-3-methylimidazolium chloride salt, pump with water at 80°C for 2 hours, and then pump with oil for 1 hour;
[0168] (3) Add 8 mL of N,N-dimethylformamide to the mixture in step (2), react for 10 min, and bathe in ice water;
[0169] (4) Add 2mL of 2-bromoisobutyryl bromide to the mixture of step (3), react at room temperature for 18h, purify the reaction product with methanol, precipitate the reaction product in methanol, and dry to obtain cellulose large molecular initiator.
[0170] (2) Preparation of guanine acrylate monomer
[0171] Weigh 15.1g of guanine, 0.811g of 2,6-di-tert-butyl-4-methylphen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com