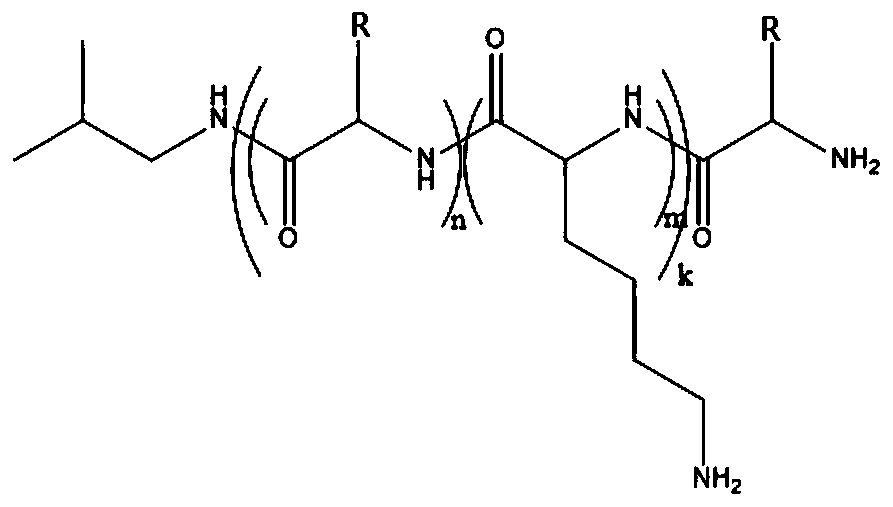
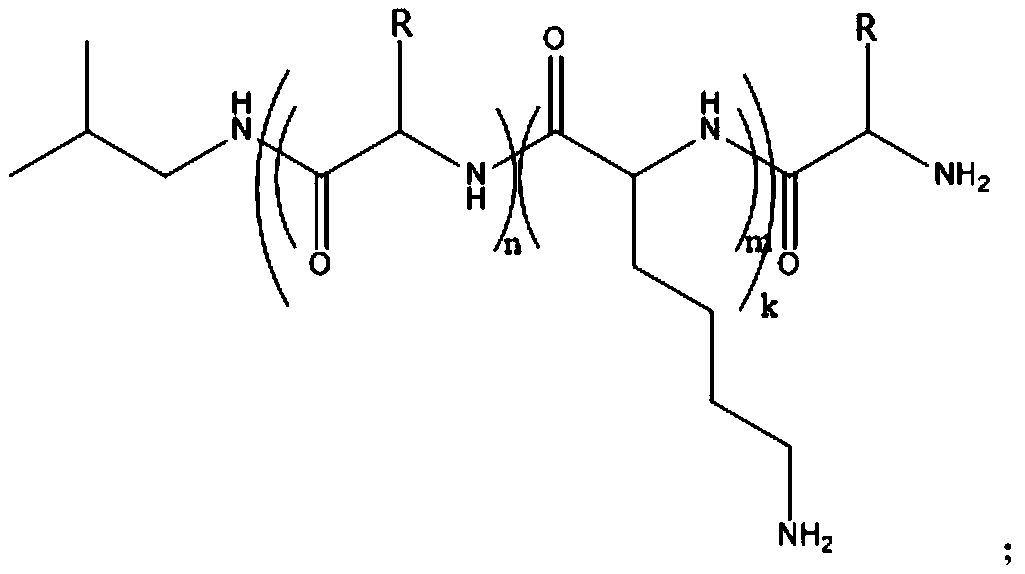
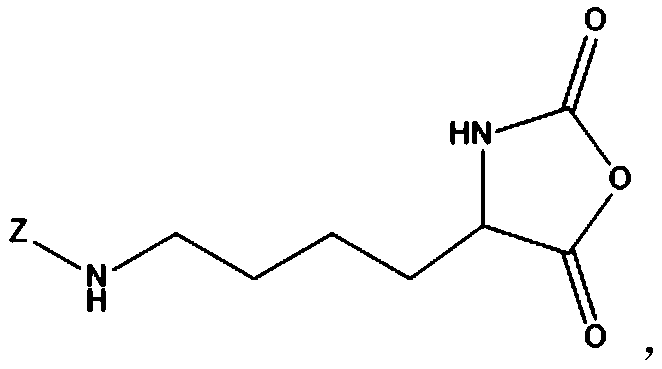
Amphiphilic multi-block antibacterial peptide copolymer, and preparation method and application thereof
A multi-block, antibacterial peptide technology, applied in the field of amphiphilic multi-block antibacterial peptide copolymer and its preparation, can solve the problems of high cost, complicated extraction process of natural antibacterial peptide, low yield and the like, and achieves low cost, Excellent broad-spectrum antibacterial properties, achieving the effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074]
[0075] The preparation method of amphiphilic multi-block class antimicrobial peptide copolymer comprises the steps:
[0076] (1), the synthesis of the hydrophilic amino acid block containing amino protecting group:
[0077] (1-1), react the hydrophilic amino acid containing the amino protecting group and triphosgene in an organic solvent to obtain the crude product of the hydrophilic amino acid-N-carboxyl-α-amino acid anhydride monomer containing the amino protecting group product, its structural formula is:
[0078]
[0079] Wherein, Z represents an amino protecting group;
[0080] (1-2) Dissolving the crude product of the hydrophilic amino acid-N-carboxy-α-amino acid anhydride monomer containing an amino protecting group in an organic solvent, and then performing recrystallization and purification in n-hexane. Repeat the steps of dissolving, precipitating and re-dissolving, and after rotary evaporation and drying, the pure product of hydrophilic amino acid-N-...
Embodiment 1
[0128] The amphiphilic triblock antimicrobial peptide copolymer K of the present embodiment 7 f 7 K 7 The preparation method comprises the steps:
[0129] (1) Add 10.0g (35.714mmol) of N(ε)-benzyloxycarbonyl-L-lysine into 50mL tetrahydrofuran, and 31.8g (107.142mmol) of triphosgene (take the amount of the above-mentioned lysine substance 3 times) within 30min dropwise into the THF solution of lysine, stirred and reacted for 8h under heating in an oil bath at 60°C, after the reaction was completed, the reaction solution was dropped dropwise into n-hexane, recrystallized and purified, and filtered Remove tetrahydrofuran to obtain the crude product of benzyloxycarbonyl (Z)-L-lysine-N-carboxyl-α-amino acid anhydride monomer; then filter the obtained benzyloxycarbonyl (Z)-L-lysine-N -Carboxyl-α-amino acid anhydride monomer was dissolved in tetrahydrofuran, and dropped dropwise into n-hexane, recrystallized for purification, and tetrahydrofuran was discarded by filtration. Repea...
Embodiment 2
[0146] The amphiphilic five-block antimicrobial peptide copolymer K of the present embodiment 5 f 5 K 5 f 5 K 5 The preparation method comprises the steps:
[0147] (1) Add 10.0g (35.714mmol) of N(ε)-benzyloxycarbonyl-L-lysine to 50mL of tetrahydrofuran, and 21.2g (71.428mmol) of triphosgene (taking the amount of the above-mentioned lysine substance) 2 times) within 30min dropwise into the tetrahydrofuran solution of lysine, stirred and reacted for 8h under heating in an oil bath at 60°C; after the reaction was completed, the reaction solution was dropped into n-hexane, recrystallized and purified, filtered and discarded Tetrahydrofuran to obtain the crude product of benzyloxycarbonyl (Z)-L-lysine-N-carboxyl-α-amino acid anhydride monomer; then filter the obtained benzyloxycarbonyl (Z)-L-lysine-N- The carboxyl-α-amino acid anhydride monomer is dissolved in tetrahydrofuran, and dropped into n-hexane drop by drop, recrystallized and purified, and the tetrahydrofuran is disc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com