Amphiphilic grafted copolymer containing antibacterial peptide, and preparation method and applications thereof
A technology of graft copolymer and antibacterial peptide, which is applied to the field of amphiphilic graft copolymer containing antibacterial peptide and its preparation, can solve the problems of high cost, low yield, complicated extraction process of natural antibacterial peptide and the like, and achieves low cost , controllable structure, excellent broad-spectrum antibacterial properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045]
[0046] The preparation method of the amphiphilic graft copolymer containing antimicrobial peptide of the present invention comprises the steps:
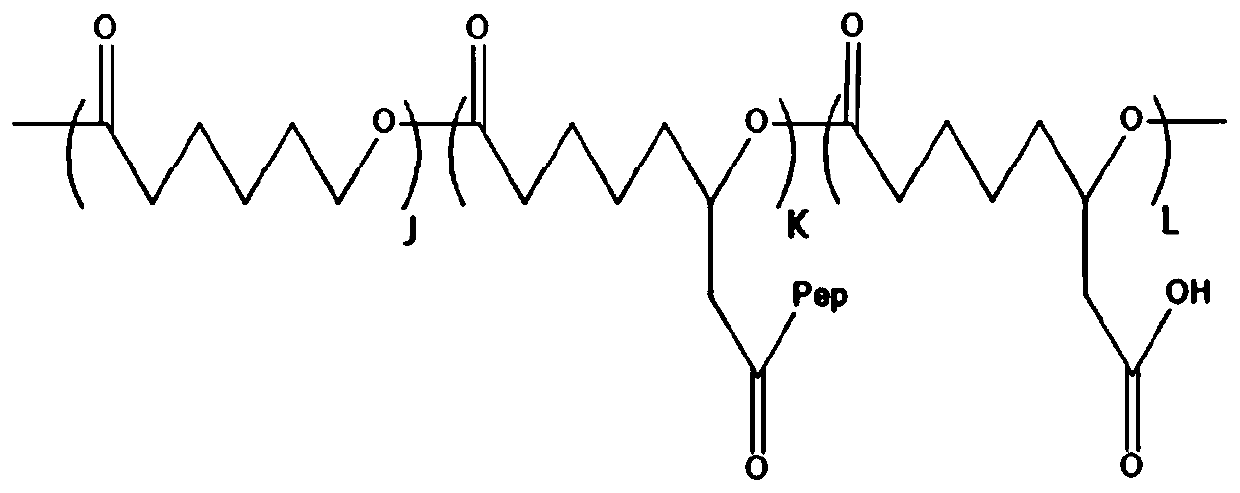
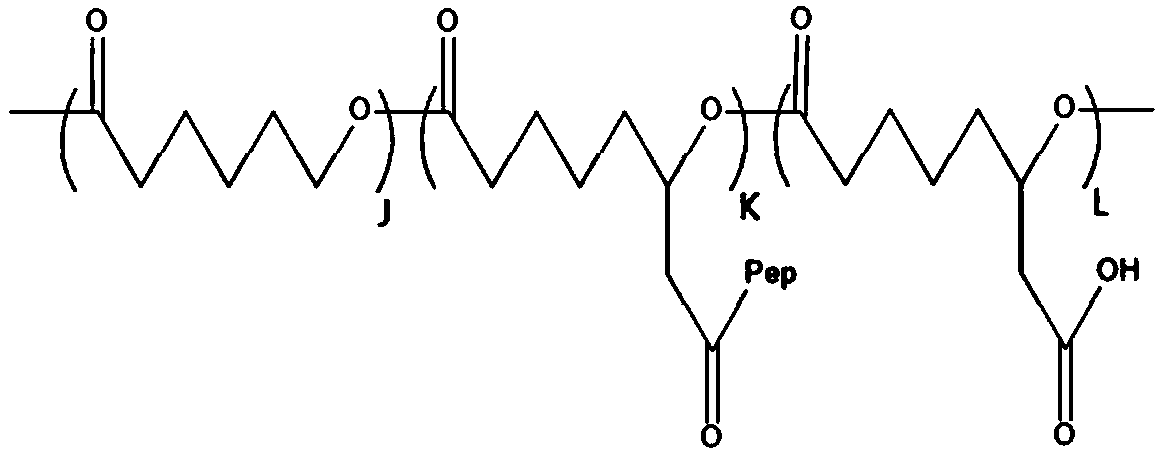
[0047] (1), with methoxypolyethylene glycol (mPEG) as a macromolecular initiator, stannous isooctanoate as a catalyst, ε-caprolactone and functionalized ε-caprolactone monomer 6-(benzyl acetate ε-caprolactone / 6-(benzyl acetate)-ε-caprolactone containing pendant benzyl acetate group by ring-opening copolymerization of BCL in organic solvent Copolymer (PCL-co-PBCL), and the benzyl protecting group was removed under the condition of palladium carbon catalytic hydrogenation, and a series of poly(ε-caprolactone) (mPEG- b-P(2-CCL-co-6-CCL)), which is a hydrophobic polymer, denoted as G, its structural formula is:
[0048]
[0049] Wherein, J and K represent the degree of polymerization of the random copolymerization block in the hydrophobic macromolecule (G) respectively;
[0050] (2) React the hydrophobic polymer, N,N'-dic...
Embodiment 1
[0072] The preparation method of the amphiphilic graft copolymer G-EPL containing ε-polylysine of the present embodiment comprises the steps:
[0073] (1) Using methoxypolyethylene glycol (mPEG) as an initiator, under the catalysis of stannous isooctanoate, ε-caprolactone and 10 g of functionalized ε-caprolactone monomer 6-(benzyl acetate Ring-opening copolymerization of ester group-ε-caprolactone) (BCL) in tetrahydrofuran to form ε-caprolactone / 6-(benzyl acetate group)-ε-caprolactone copolymer with pendant benzyl acetate group (PCL-co-PBCL). Then, the benzyl protecting group of PCL-co-PBCL was removed by palladium / carbon catalytic hydrogenation, and finally poly(ε-caprolactone) (PCL-co-PCCL) containing pendant ethyl carboxyl functional groups was obtained, which was highly hydrophobic. Molecule, denoted as G, its structural formula is:
[0074]
[0075] Wherein, J and K represent the degree of polymerization of the random copolymerization block in the hydrophobic macromo...
Embodiment 2
[0085] The preparation method of the amphiphilic graft copolymer G-melittin containing melittin of the present embodiment comprises the steps:
[0086] (1) Using methoxypolyethylene glycol (mPEG) as an initiator, under the catalysis of stannous isooctanoate, ε-caprolactone and 10 g of functionalized ε-caprolactone monomer 6-(benzyl acetate Ring-opening copolymerization of ester group-ε-caprolactone) (BCL) in tetrahydrofuran to form ε-caprolactone / 6-(benzyl acetate group)-ε-caprolactone copolymer with pendant benzyl acetate group (PCL-co-PBCL). Then, the benzyl protecting group of PCL-co-PBCL was removed by palladium / carbon catalytic hydrogenation to obtain poly(ε-caprolactone) (PCL-co-PCCL) containing pendant ethyl carboxyl functional groups, which is a hydrophobic polymer. Denoted as G, its structural formula is:
[0087]
[0088] Wherein, J and K represent the degree of polymerization of the random copolymerization block in the hydrophobic macromolecule (G) respectively...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com