Guanidyl hyaluronic acid type antibacterial hydrogel as well as preparation method and application thereof
A hyaluronic acid and hydrogel technology, applied in bandages, medical science, etc., can solve the problems of skin wounds susceptible to bacterial infection, wounds difficult to heal, and body fluid loss, etc., to achieve good mechanical properties and rheological properties, and stable structure , the effect of high swelling performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
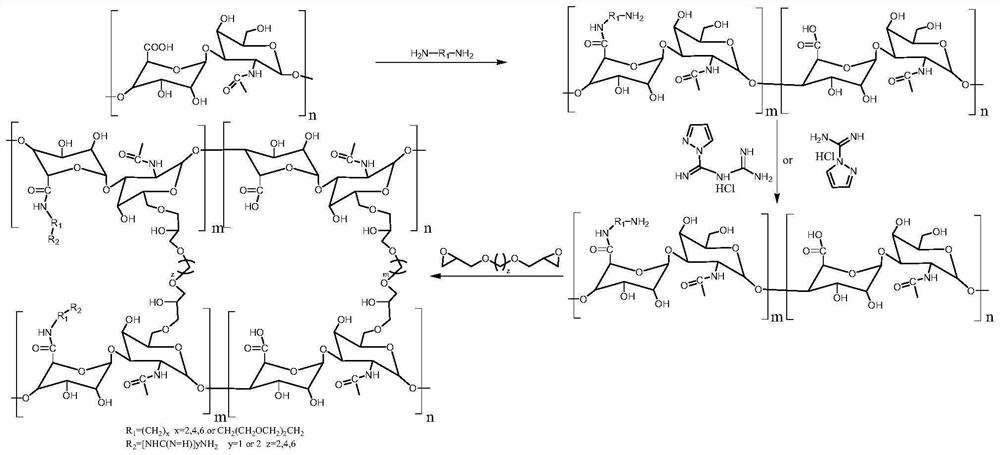
[0043] Embodiment 1: the preparation method of ethylamine monoguanidine hyaluronic acid gel (HA-ESG-Gel)
[0044] Step S1: ethylamine hyaluronic acid (HA-E-NH 2 )Synthesis:
[0045] At room temperature, 4g of sodium hyaluronate (12k Da HA, wherein the amount of carboxyl group is 10mmol) was dissolved in 40ml of deionized water, and stirred evenly; another 600mg of ethylenediamine (10mmol) was dissolved in 10ml of pure water, and This was added to the aforementioned HA solution. Adjust the pH value of the reaction mixture to 7.5 with 1M HCl solution, stir for later use; weigh 2.304g of EDC (12mmol) and 1.380g of NHS (12mmol) and dissolve them in 10ml of pure water and mix well. Slowly add the pH-adjusted HA and ethylenediamine mixed solution dropwise into the mixed aqueous solution of EDC and NHS, stir evenly under a magnetic stirrer, use 1M NaOH solution to keep the pH value of the reaction system at about 7.5, and react at room temperature for 24 hours . After the reactio...
Embodiment 2
[0050] Embodiment 2: Preparation of ethamformin hyaluronic acid gel (HA-EBG-Gel)
[0051] Step S1: ethylamine hyaluronic acid (HA-E-NH 2 )Synthesis:
[0052] At room temperature, 10g of sodium hyaluronate (260k Da HA, the amount of carboxyl group is 25mmol) was dissolved in 100ml of formamide, stirred evenly, another 1.8g of ethylenediamine (30mmol) was dissolved in 10ml of pure water, and It was added dropwise in the HA solution; the pH value of the reaction mixture was adjusted to 7.5 with 1M HCl solution, and stirred for subsequent use; the DCC (30mmol) of 4.518g and the NHS (30mmol) of 3.467g were dissolved in 20ml of formamide respectively and Mix evenly; slowly add the pH-adjusted HA and ethylenediamine mixed solution into the mixed aqueous solution of DCC and NHS, stir evenly under a magnetic stirrer, and use 1M NaOH solution to keep the pH of the reaction system at about 7.5. React at 40°C for 12 hours; after the reaction, settle the reaction solution in absolute eth...
Embodiment 3
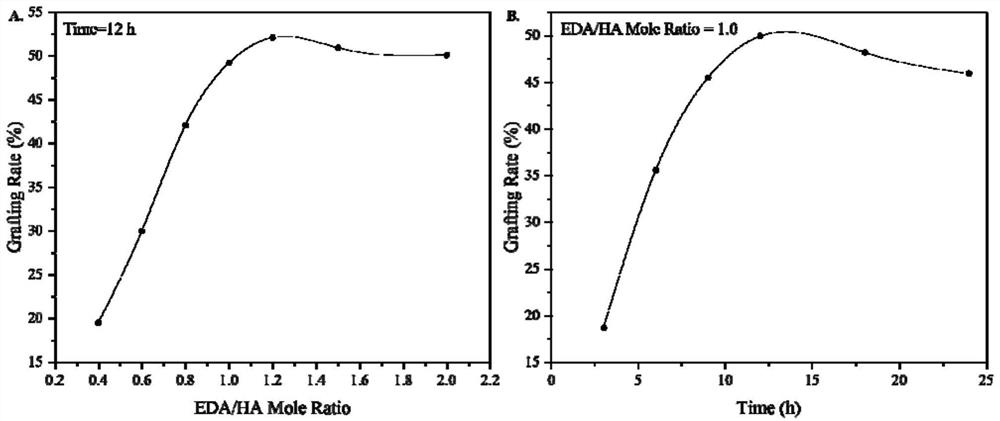
[0073] Embodiment 3: the influence of the mol ratio of ethylenediamine (EDA) and HA on guanidino grafting rate
[0074] A preparation method of ethylamine monoguanidine hyaluronic acid (HA-ESG), the reaction time is set at 12h, the amount of EDA is adjusted in step S1, so that the molar ratio of EDA to HA carboxyl is 0.4-2.0, specifically:
[0075] Step S1: 1.2g of hyaluronic acid (HA, 10K Da, the amount of carboxyl group is 3mmol) was completely dissolved in 10mL ultrapure water to obtain HA aqueous solution; then 680mg of dimethylaminopropylethylcarbodiimide salt acid salt (EDC·HCl, 3.6mmol) and 480mg of N-hydroxysuccinimide (NHS, 3.6mmol) were added to the HA aqueous solution in batches, stirred at room temperature for 1h; then 72mg to 360mg of ethylenediamine (1.2 ~6mmol), stirred and reacted at 25°C for 12h; after the reaction, the reaction solution was settled in absolute ethanol, the precipitate was washed twice with absolute ethanol, filtered, washed, and freeze-dried ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com