Special catalyst for reaction for preparing synthesis gas through dry reforming of methane, and preparation method thereof
A methane dry reforming and catalyst technology, which is applied in hydrogen/syngas production, chemical instruments and methods, chemical elements of heterogeneous catalysts, etc., can solve problems such as multiple side reactions, catalyst carbon deposition, deactivation, etc. Superior, solve the effect of high temperature sintering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A. Dissolve 8.66g of lanthanum nitrate, 6.46g of tetrabutyl titanate and 0.429g of zirconium nitrate in 50ml, 47.5ml and 2.5ml of absolute ethanol respectively. After completely dissolving, mix the three salt solutions in a beaker, and Sonicate at 25k Hz for 10 minutes.
[0031] B. be that 1wt.% is added the rhodium nitrate solution that 0.65g concentration is 1.13M in the solution of step A by rhodium in the final catalyst theoretical load, then add 50g concentration and be the dehydrated alcohol solution of the citric acid of 0.65M Stir evenly, and then sonicate at 25k Hz for 1 h to mix evenly to obtain a mixed solution.
[0032] C. Stir the mixed solution obtained in step B at room temperature under the condition of magnetic stirring for 10 h. Then, rotary evaporation was carried out at 60° C. in a vacuum environment until the solvent was evaporated to dryness to obtain a catalyst precursor.
[0033] D. Dry the catalyst precursor obtained in step C at 100°C for 12h...
Embodiment 2
[0037] A. Dissolve 8.66g of lanthanum nitrate, 3.4g of tetrabutyl titanate and 4.29g of zirconium nitrate in 50ml, 25ml and 25ml of absolute ethanol respectively. Hertzian ultrasound for 10 minutes.
[0038] B. be that 1wt.% is added the rhodium nitrate solution that 0.65g concentration is 1.13M in the solution of step A by the theoretical load of rhodium in the final catalyst, then add 50g concentration and stir the dehydrated ethanol solution that is the citric acid of 0.65M homogeneously, and then ultrasonicated at 25k Hz for 1h to mix uniformly to obtain a mixed solution.
[0039] C. Stir the mixed solution obtained in step B at room temperature under the condition of magnetic stirring for 10 h. Then, rotary evaporation was carried out at 60° C. in a vacuum environment until the solvent was evaporated to dryness to obtain a catalyst precursor.
[0040] D. Dry the catalyst precursor obtained in step C at 100°C for 12h, and then calcinate it in a muffle furnace at 900°C fo...
Embodiment 3
[0044]Step A dissolves 8.66g of lanthanum nitrate in 25ml of absolute ethanol, and others are the same as in Example 1;
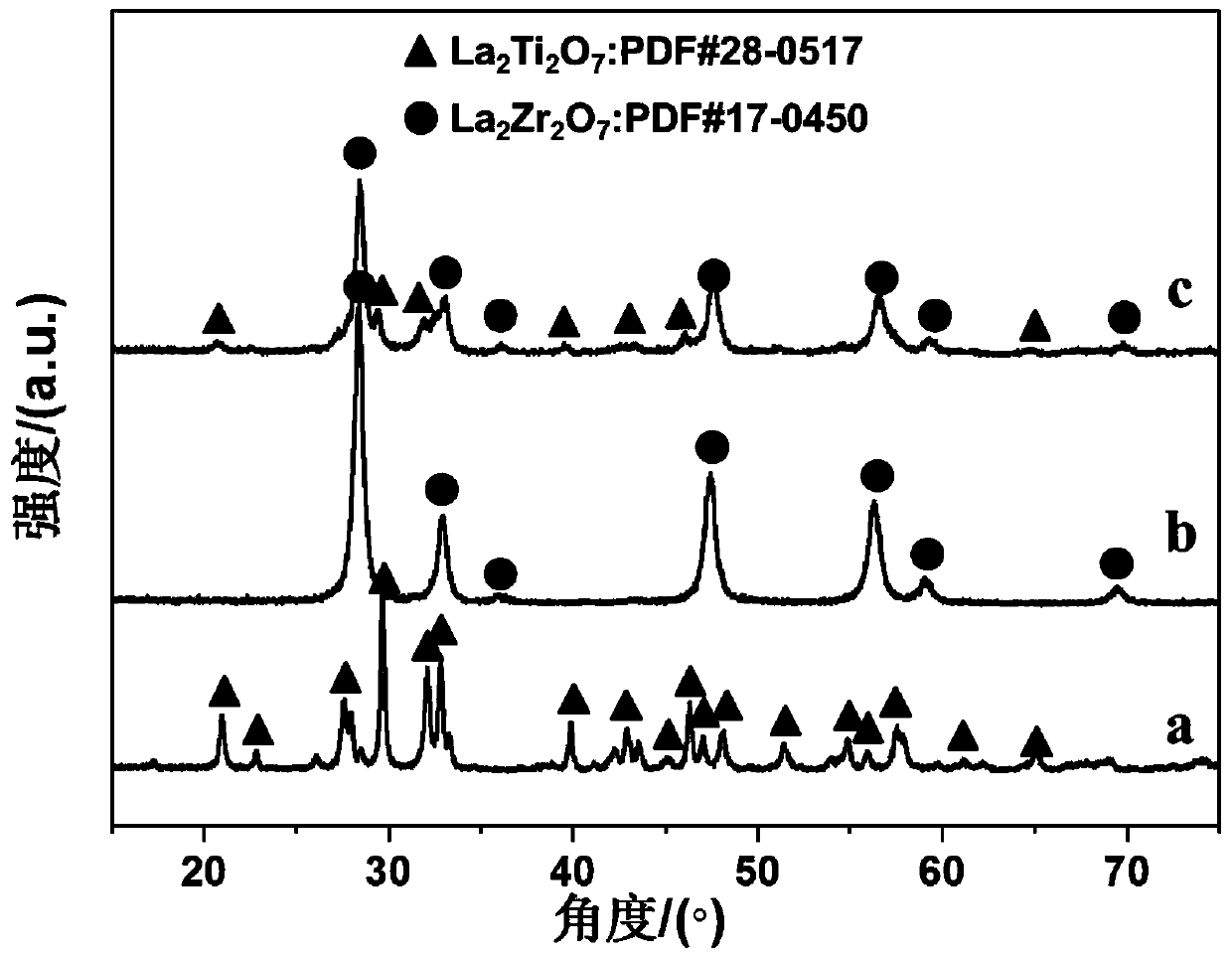
[0045] Step B-D is also with embodiment 1, obtains catalyst Rh / 0.95La 2 Ti 2 o 7 -0.05La 2 Zr 2 o 7 . The loaded amount of Rh was measured to be 1.30 wt.% by ICP.
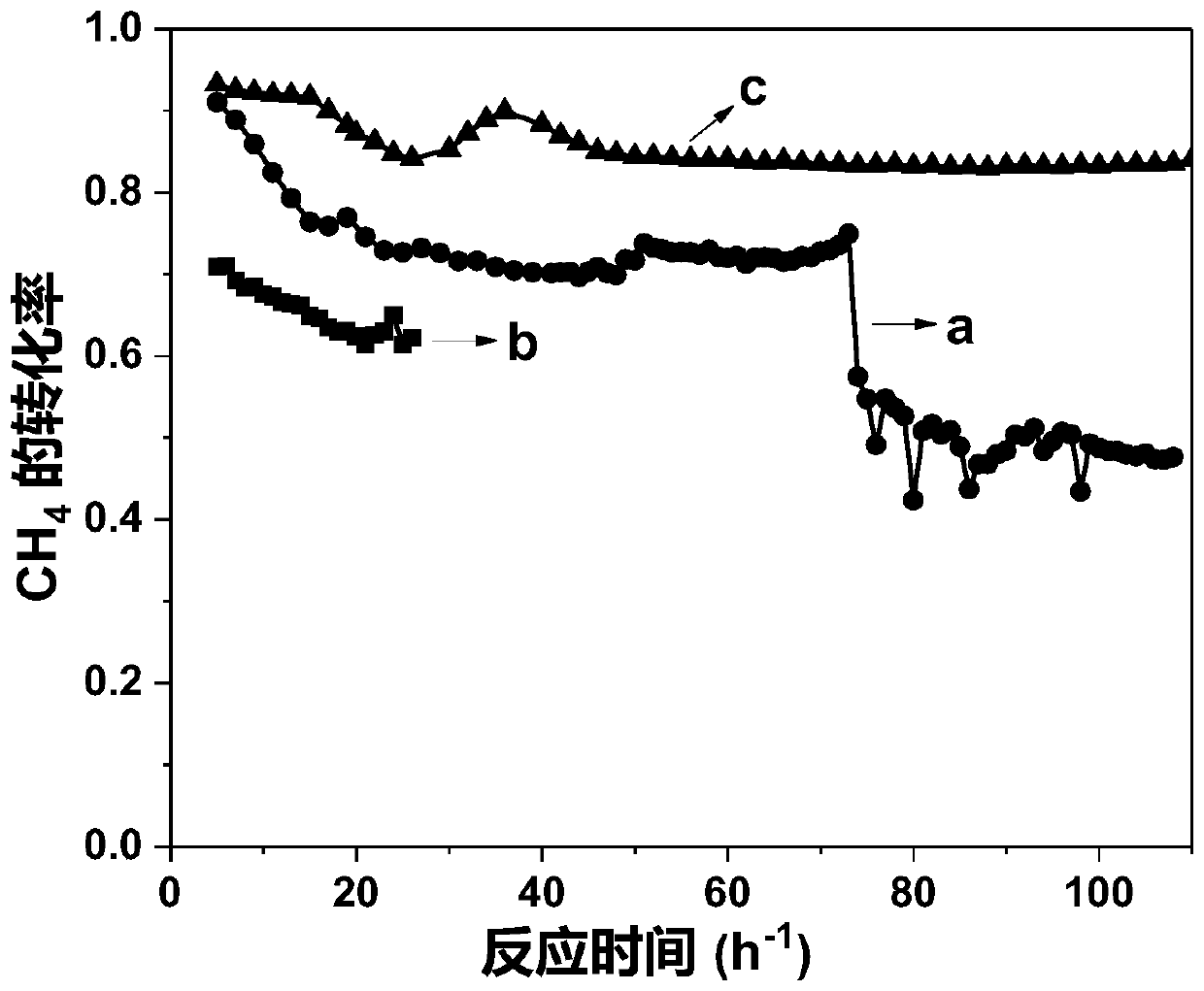
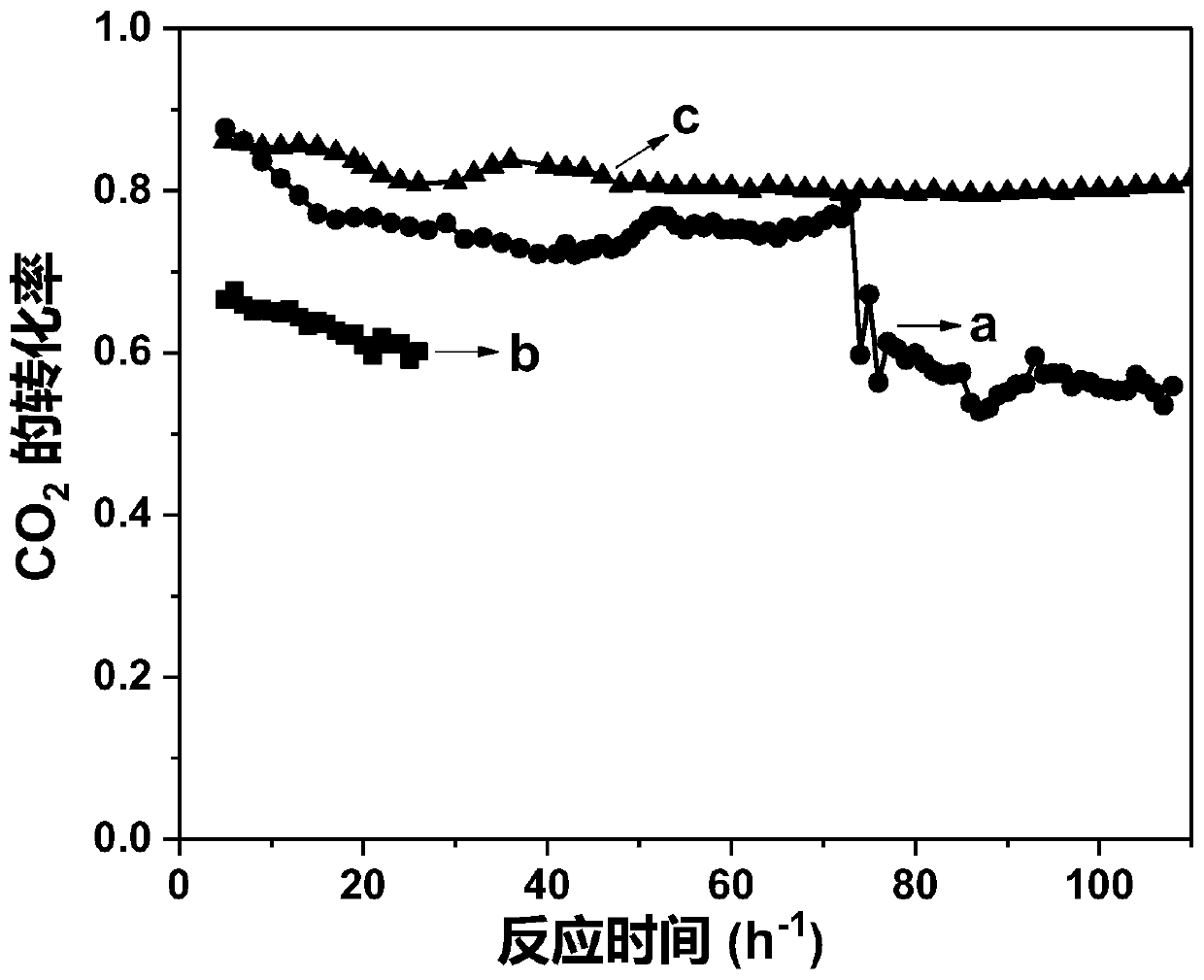
[0046] The performance in different temperature stages in the reaction of methane dry reforming to synthesis gas was measured by the method of implementation 1, and the results are shown in Table 1-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com