Novel tag protein for protein enrichment expression and intracellular localization as well as application thereof
A technology for labeling proteins and proteins, applied in the field of labeling proteins, can solve the problems of reducing cell activity, limiting protein yield, increasing production costs, etc., to achieve the effects of increasing final output, maintaining activity, and reducing loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1. Expression of pEGFP-C1 and pEGFP-C1-RA1 in 293T cells
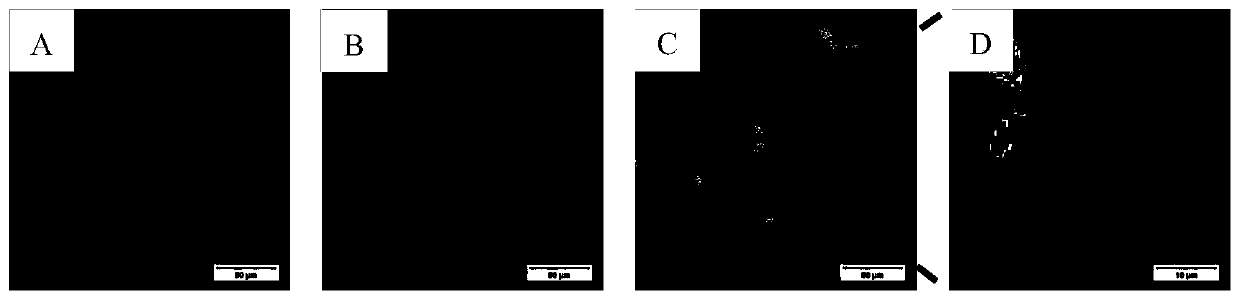
[0060] figure 1 . Confocal microscopy results of 293T cells transfected with pEGFP-C1 for 24 hours.
[0061] A) Excitation wavelength 488, signal collection channel 507, eGFP green fluorescence signal;
[0062] B) Excitation wavelength 364, signal collection channel 461, DAPI blue fluorescence signal;
[0063] C) Experimental results after fusion of eGFP and DAPI signaling channels;
[0064] D) Partial magnification of 5 times.
[0065] from figure 1 In A, it can be seen that there are a large number of green fluorescent signals (derived from the expression of eGFP) in the 293T cells successfully transfected with the pEGFP-C1 plasmid. figure 1 The blue signal in B comes from DAPI. Due to the use of a membrane-breaking agent (TritonX-100), no matter whether the cells are successfully transfected and express eGFP, the nuclei can be stained by DAPI, so the number of cells is greater than figure 1 a. ...
Embodiment 2
[0073] Example 2. Expression of pEGFP-C1 and pEGFP-C1-RA1 in Hela cells
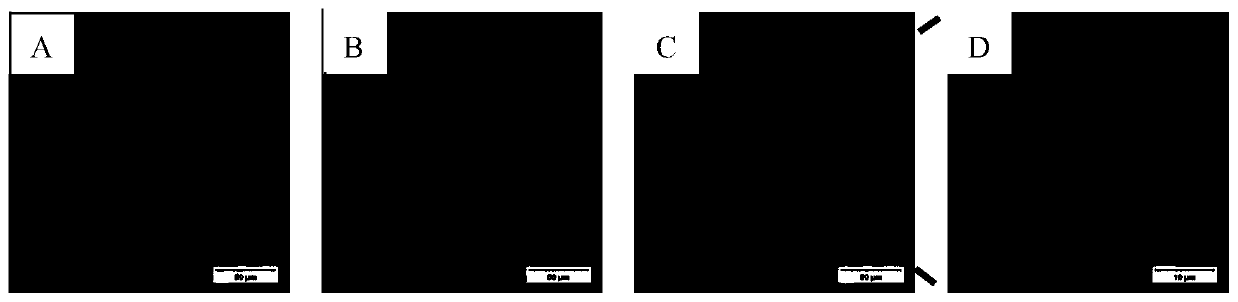
[0074] from image 3 In A, it can be seen that there are a large number of green fluorescent signals (derived from the expression of eGFP) in the Hela cells successfully transfected with the pEGFP-C1 plasmid. image 3 The blue signal in B comes from DAPI. Due to the use of a membrane-breaking agent (TritonX-100), no matter whether the cells are successfully transfected and express eGFP, the nuclei can be stained by DAPI, so the number of cells is greater than image 3 a. Will image 3 After the images of A and 3B are fused, it can be found that the cells emitting eGFP green fluorescence overlap with some DAPI-stained nuclei. After partially amplifying the overlapping part (5 times), it can be found that the green fluorescent signal is evenly distributed in Hela cells successfully transfected with pEGFP-C1 plasmid, and the DAPI signal in the nucleus is blocked to a certain extent.
[0075] image 3 ....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com