Method for removing organic micro-pollutants from drinking water by using persulfate reinforced iron salt coagulation process
A persulfate and pollutant technology, applied in the field of water treatment, can solve the problems of secondary pollution, high toxicity, slow conversion rate, etc., achieve the best removal effect, good effluent quality, and low treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
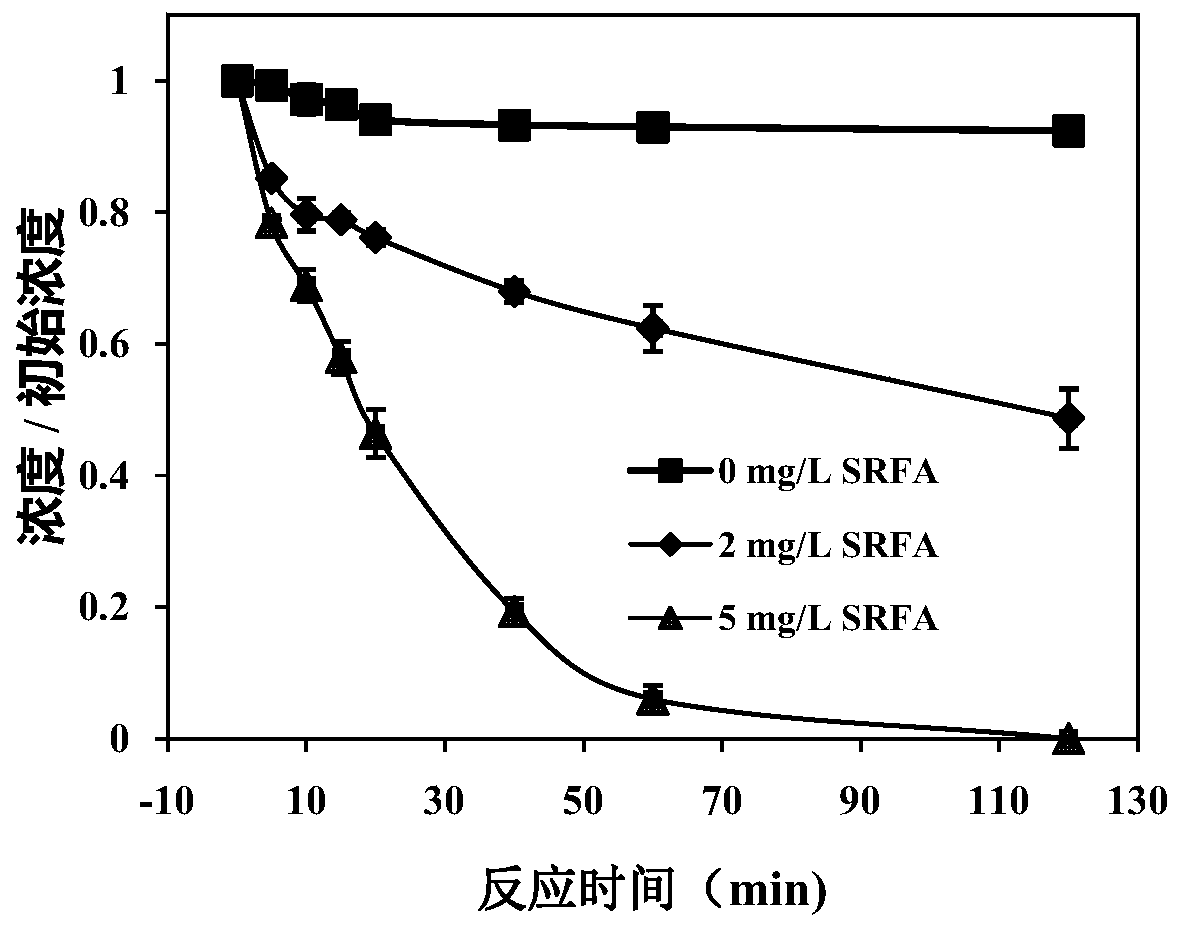
[0041] Example 1 Using Suwannee River Fulvic Acid to Synergize with Ferric Chloride to Activate Sodium Peroxodisulfate to Degrade Carbamazepine
[0042] 1. Utilize Suwannee River Fulvic Acid (SRFA) (organic matter in water, purchased from the International Humic Acid Association) to activate ferric chloride to activate sodium peroxodisulfate to degrade carbamazepine, including the following steps:
[0043] Treatment group 1: Prepare 150 mL of carbamazepine aqueous solution with a concentration of 1 μM, add 2 mg / L SRFA (as a model fulvic acid, purchased from the International Humic Acid Association), 0.02 mM FeCl 3 and 0.2mM sodium peroxodisulfate, the above solution was stirred and reacted at 30rpm. The concentration change of carbamazepine was determined during the reaction.
[0044] Treatment group 2: The concentration of SRFA added was 10 mg / L, and other experimental conditions were the same as treatment group 1.
[0045] Control group: no SRFA was added, and other experi...
Embodiment 2
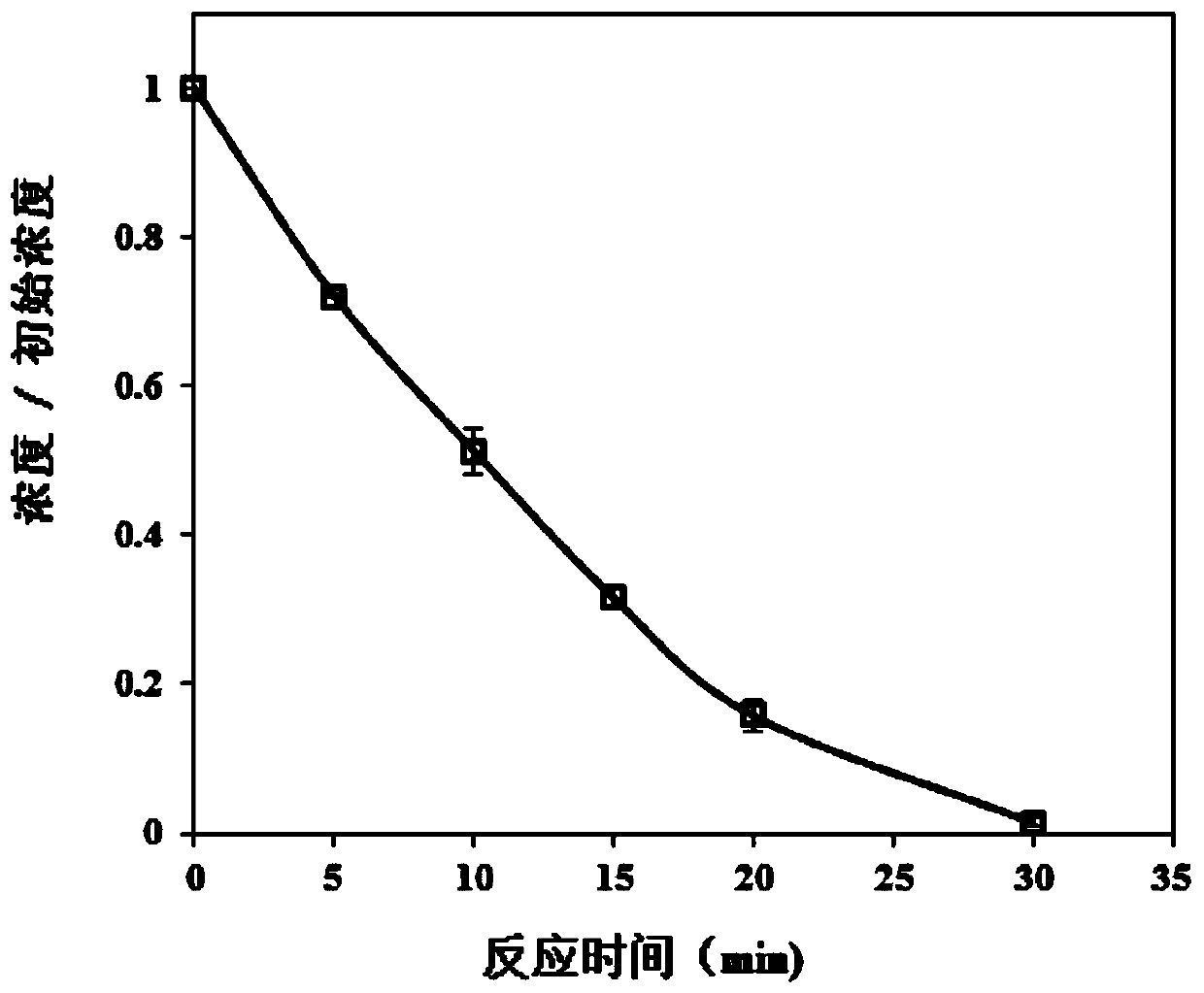
[0048] Example 2 Degradation of Bisphenol A by Activation of Potassium Monopersulfate with Humic Acid and Ferric Sulfate
[0049] 1. A method for degrading bisphenol-A by using humic acid in conjunction with ferric sulfate to activate potassium hydrogen persulfate to degrade bisphenol A, comprising the steps:
[0050] Prepare 500 mL of bisphenol A solution with a concentration of 0.4 μM, add 8 mg / L humic acid (purchased from sigma), and mix evenly by hand; prepare a certain concentration of ferric sulfate solution and potassium monopersulfate solution, and mix the two in advance Then it is added into the aqueous solution containing bisphenol A, so that the concentrations of iron ions and potassium monopersulfate in the reaction system are respectively 0.2 and 0.3 mM. The above solutions were mixed evenly, and placed in a shaker for 30 minutes of shaking reaction, and the change of the concentration of bisphenol A was measured during the reaction.
[0051] 2. Experimental resu...
Embodiment 3
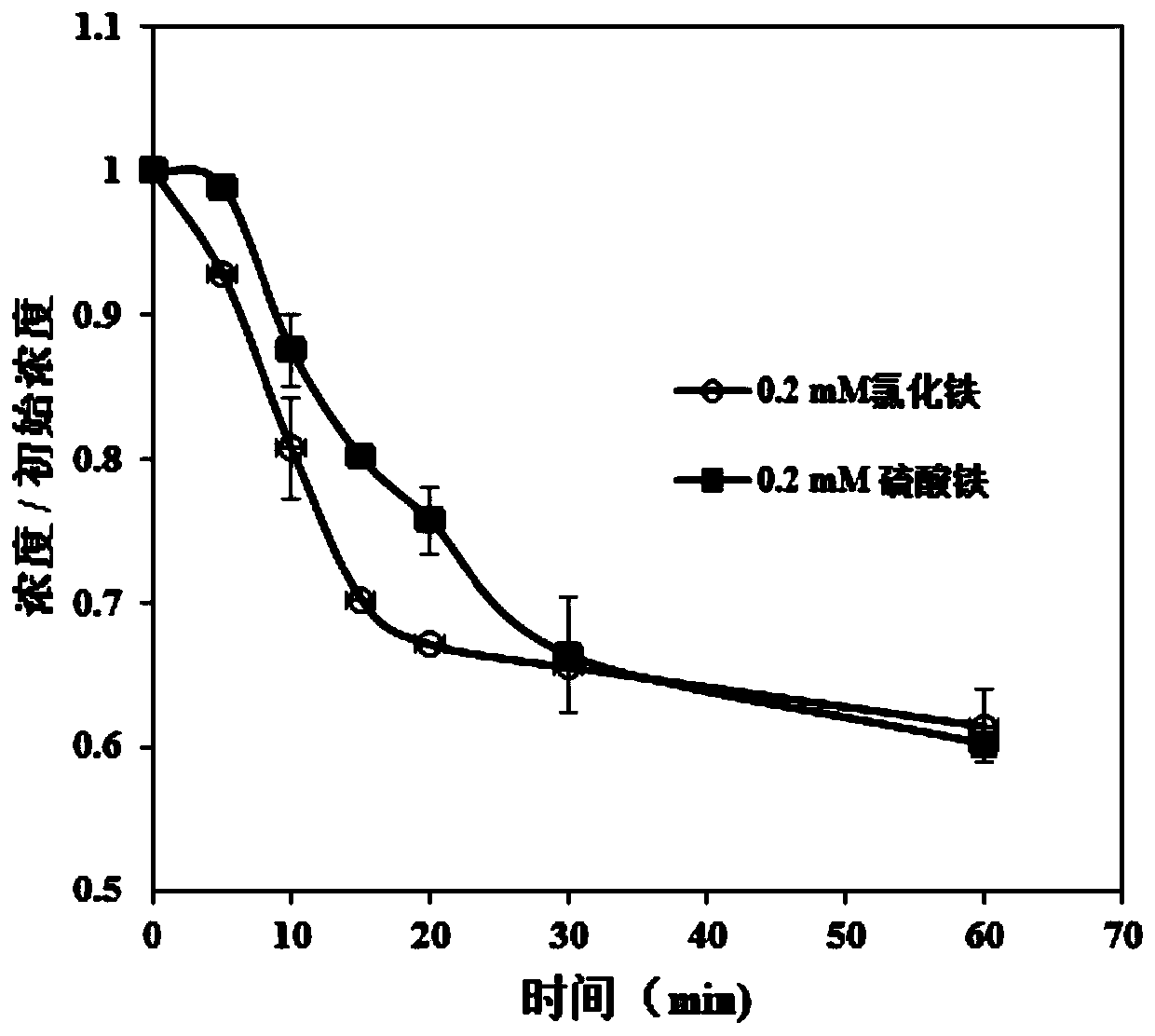
[0053] Example 3 Potassium monopersulfate strengthens iron salt coagulation process to remove carbamazepine in natural water bodies
[0054] 1. Utilize potassium hydrogen persulfate to strengthen iron-salt coagulation process to remove carbamazepine in natural water bodies, including the following steps:
[0055] Add 800mL of natural water (dissolved organic matter = 1.7mg / L, pH = 7.5, turbidity = 14.7NTU) into two 1L coagulation buckets, add a small amount of carbamazepine solution to make the concentration 1μM; Add 0.2mM ferric chloride or ferric sulfate, and add 1mM potassium hydrogen persulfate at the same time, stir at 200rpm for 1min, stir at 30rpm for 30min, and let it stand for 30min to precipitate for a reaction. The concentration of carbamazepine in water was determined at different reaction times. And after the reaction, the pH value, turbidity and iron ion concentration of the water body were measured.
[0056] 2. Experimental results
[0057] as attached imag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com