A sesquiterpene lactone compound and its preparation method and its application in the preparation of drugs for preventing and treating nasopharyngeal carcinoma
A technology of ester compounds and sesquiterpenes, which is applied in the field of compounds and medicines, can solve the problems of easy metastasis and recurrence, poor patient treatment compliance, and no specific anti-nasopharyngeal carcinoma, so as to inhibit the proliferation of nasopharyngeal carcinoma cells and promote Effects of Cancer Cell Apoptosis, Migration and Invasion Inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
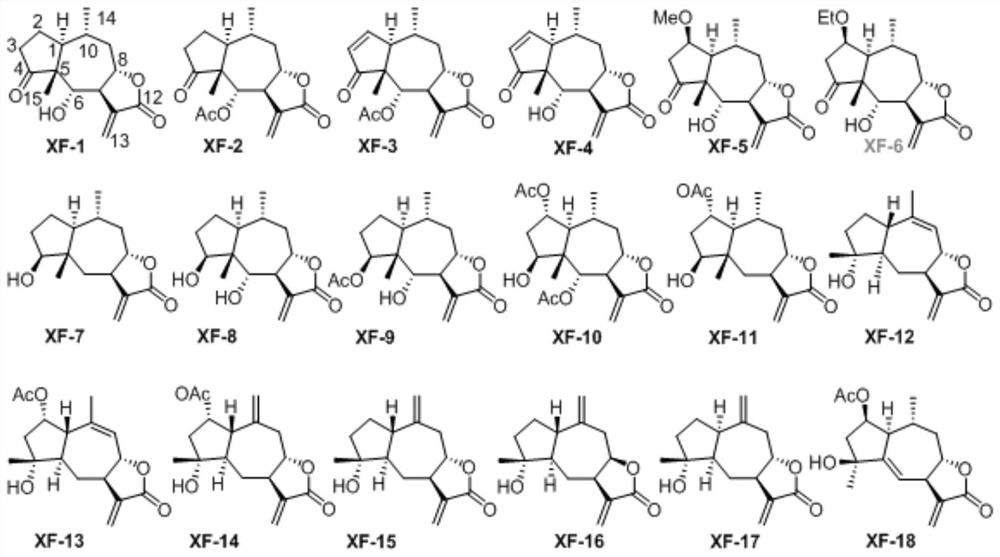
[0050] Compounds XF-1 to XF-18 were prepared from Inula flower (Euphorbia antiquorum L.).
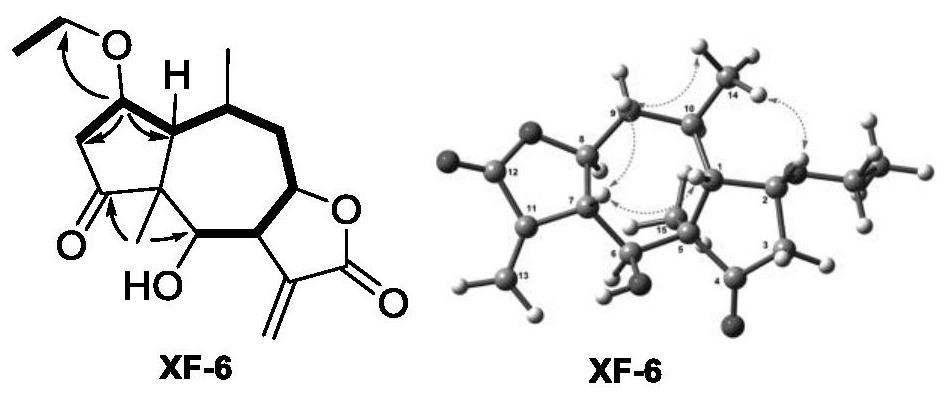
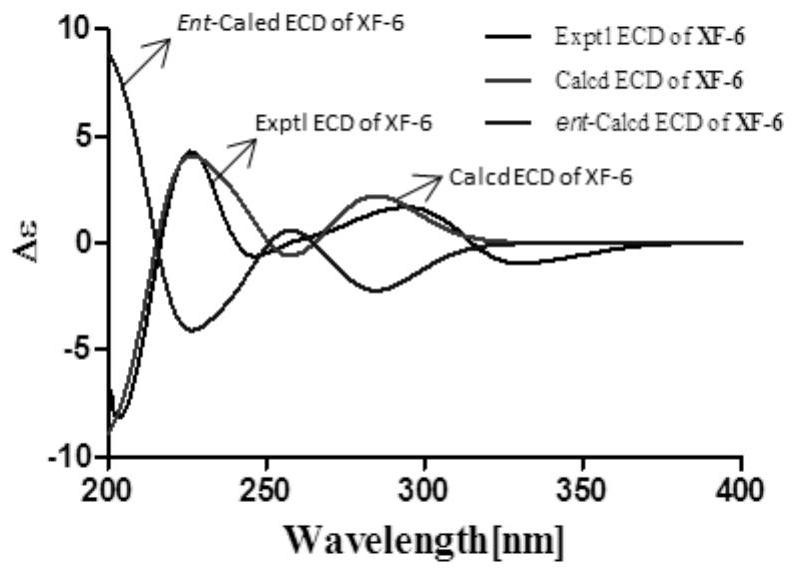
[0051] The dried inflorescences of Inula inula were extracted three times with 95% ethanol water at room temperature, each time for 72 hours, and the extract was concentrated under reduced pressure to obtain a crude extract. The crude extract was suspended in water, extracted with ethyl acetate, and concentrated under reduced pressure to obtain the ethyl acetate fraction, which was chromatographed on a silica gel column (100-200 mesh) and eluted with petroleum ether / ethyl acetate gradient (9:1, 8:2,2:1,1:2,0:1), after TLC detection, it was divided into 3 components (A-C). Part B (54 g) was subjected to reverse phase column chromatography, eluting with methanol / water (50%-100%) to obtain seven fractions Fr.B1-7. Fr.B2 was eluted with Sephadex LH-20 with dichloromethane / methanol (1:1) to obtain compounds XF-1 (120mg), XF-2 (2.3g), XF-3 (50mg), XF-4 (20mg). Fr.B3 was separated by HPLC t...
Embodiment 2
[0053] Preparation of Ergolide Derivatives XF2-1~XF2-30
[0054] Synthetic route a: at room temperature, the compound Ergolide (XF2, 10mg, 0.03mmol) and the catalytic equivalent of 10% Pd / C were dissolved in 2mL of methanol, in H 2 Under conditions, stir for 1h. The completion of the reaction was monitored by low-resolution mass spectrometry, the device was removed, Pd / C was removed by filtration with a microporous membrane, and concentrated under reduced pressure to obtain white solid XF2-1 (10 mg, 100%).
[0055] b: Dissolve the compound Ergolide (50mg, 0.17mmol) in tetrahydrofuran (2mL), stir in an ice bath, add 5e.q lithium hydroxide (20mg, 0.85mmol) dissolved in 1mL ethanol / water (1 / 1), TLC Monitor the reaction. After the reaction is complete, spin dry and add an appropriate amount of saturated saline, add concentrated hydrochloric acid dropwise in an ice bath, and precipitate a white solid Carpesiolin (XF-1, 37mg, 85%)
[0056] c: Put the compound Ergolide (20mg, 0.08m...
Embodiment 3
[0064] Prepare formula (I) earlier by the method of embodiment 1 and 2, add water for injection according to routine, fine filter, potting sterilization makes injection solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com