Method for prokaryotic expression of recombinant chicken angiopoietin-like protein 4 and application thereof
A prokaryotic expression and protein technology is applied in the field of prokaryotic expression of recombinant chicken angiopoietin-like protein 4, which can solve the problems of unsuccessful expression of recombinant chicken ANGPTL4 protein and low sequence homology of ANGPTL4 protein.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
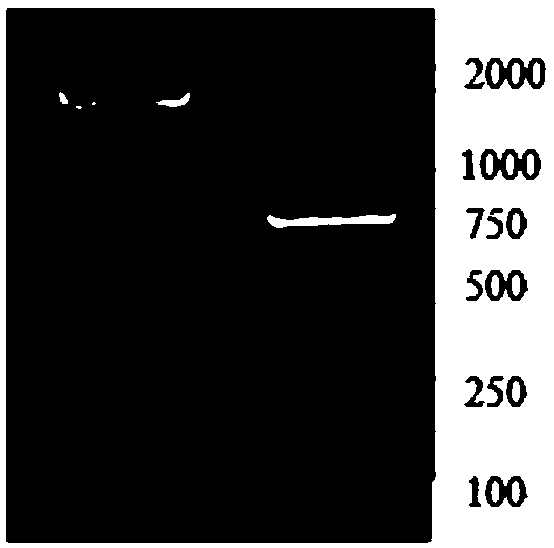
[0057] Embodiment 1, the preparation of recombinant chicken ANGPTL4 protein
[0058] Use UniProt to find the amino acid sequence of chicken ANGPTL4 (serial number F1NUQ4) and the signal peptide part (1-18 amino acids), remove the signal peptide and add the His-SUMO tag to the N-terminus, compare the preferred codons of E. coli, according to the codons On the basis of not changing the amino acid composition and arrangement sequence, the gene sequence encoding His-SUMO-ANGPTL4 was transformed and replaced with codons preferred by Escherichia coli to obtain a new gene sequence encoding His-SUMO-ANGPTL4 (SEQ ID NO.2), its protein amino acid sequence is SEQ ID NO.4, sent to Yingwei Jieji (Shanghai) Trading Co., Ltd. for whole gene synthesis, and constructed into pET21a with Nde I and Xho I restriction sites ( +) in the expression vector.
[0059] 2. Transformation of expression strains
[0060] The recombinant prokaryotic expression plasmid pET21a-His-SUMO-ANGPTL4 was transformed...
Embodiment 2
[0077] Example 2, Effects of different concentrations of recombinant chicken ANGPTL4 protein on broiler blood biochemical indicators
[0078] 1. Experimental design
[0079] Thirty-six AA meat roosters with similar body weight at 35 days of age were selected in the fasting state (fasting for 12 hours), and were randomly divided into 6 treatments, with 6 repetitions for each treatment, and 1 chicken for each repetition. Each group was treated with intravenous injection of 0, 20, 100, 500, 2500 and 12500 ng / kg of recombinant chicken ANGPTL4 protein according to the body weight of the broiler, and the fasting blood of the test broiler was collected 30 minutes after the injection, the serum was separated, stored at -20°C, and measured by an automatic biochemical analyzer Blood biochemical indicators such as triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) and low-density lipoprotein (LDL), and the content of very low-density lipoprotein (VLDL) by enzyme-li...
Embodiment 3
[0088] Embodiment 3, the influence of recombinant chicken ANGPTL4 protein on fat metabolism of broiler skeletal muscle
[0089] How to increase the fat content of broiler skeletal muscle in a targeted manner is a major scientific problem in the current poultry industry. Studies have found that the addition of Clostridium butyricum can achieve this goal, but its molecular mechanism has not yet been clarified. ANGPTL4 is a protein closely related to animal skeletal muscle fat metabolism, and is an important medium connecting intestinal microorganisms and host skeletal muscle fat metabolism. The previous inventor's animal experiments found that Clostridium butyricum can cause ANGPTL4 in the ileum and serum of broiler chickens. Therefore, it is speculated that ANGPTL4 plays an important role in the directional increase of fat content in broiler skeletal muscle by Clostridium butyricum. In order to further confirm this hypothesis, this experiment partly takes prokaryotic expression...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com