Application of B cell vaccine in preparation of drugs for treating rheumatoid arthritis
A B-cell and arthritis technology, applied in the field of B-cell vaccines, can solve problems such as limited selection, unsatisfactory vaccine treatment effect, and inability to cure, and achieve low frequency of administration, reduce bone erosion, and have no immune tolerance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1CD19
[0034] Example 1CD19 + Preparation of total B cell vaccine
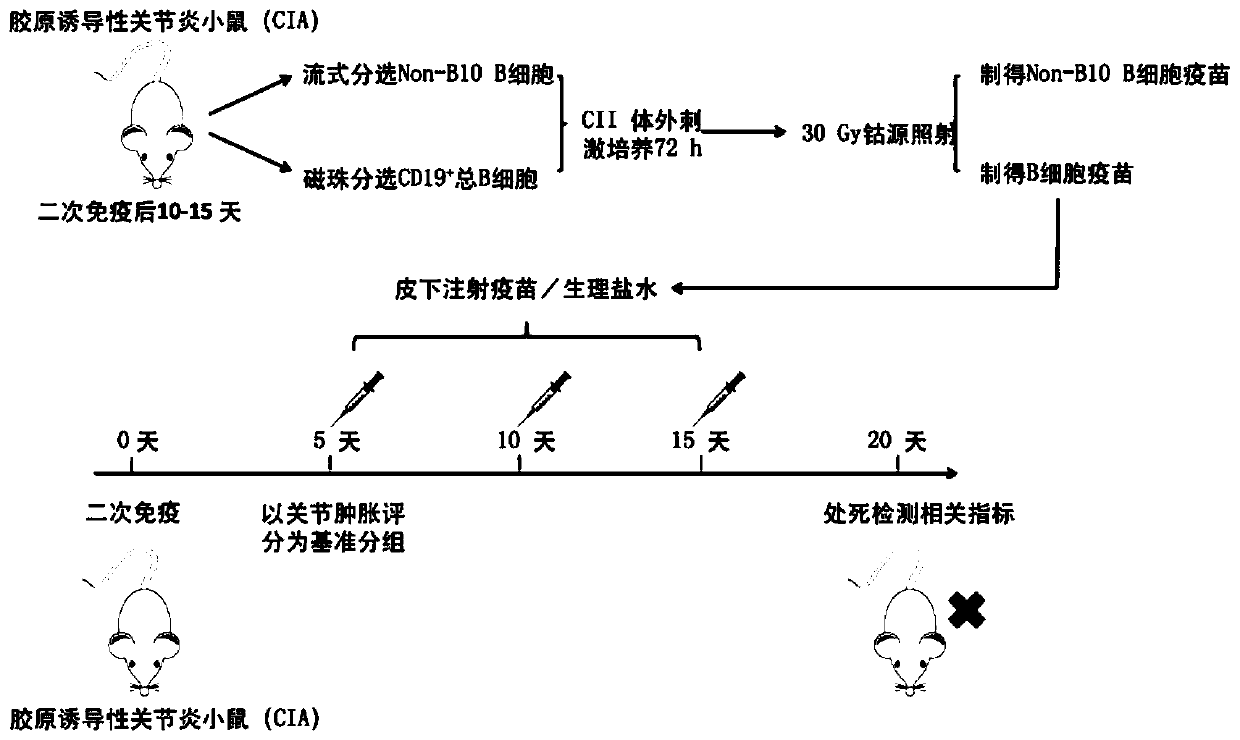
[0035] Experimental animals: Specific pathogen free (SPF) DBA / 1 male mice of 6-8 weeks purchased from Beijing Huafukang Biotechnology Co., Ltd., all of which were fostered in the SPF-level environment of the Animal Laboratory of Peking University People's Hospital. Modeling experiment was carried out after 1 week of adaptive feeding.
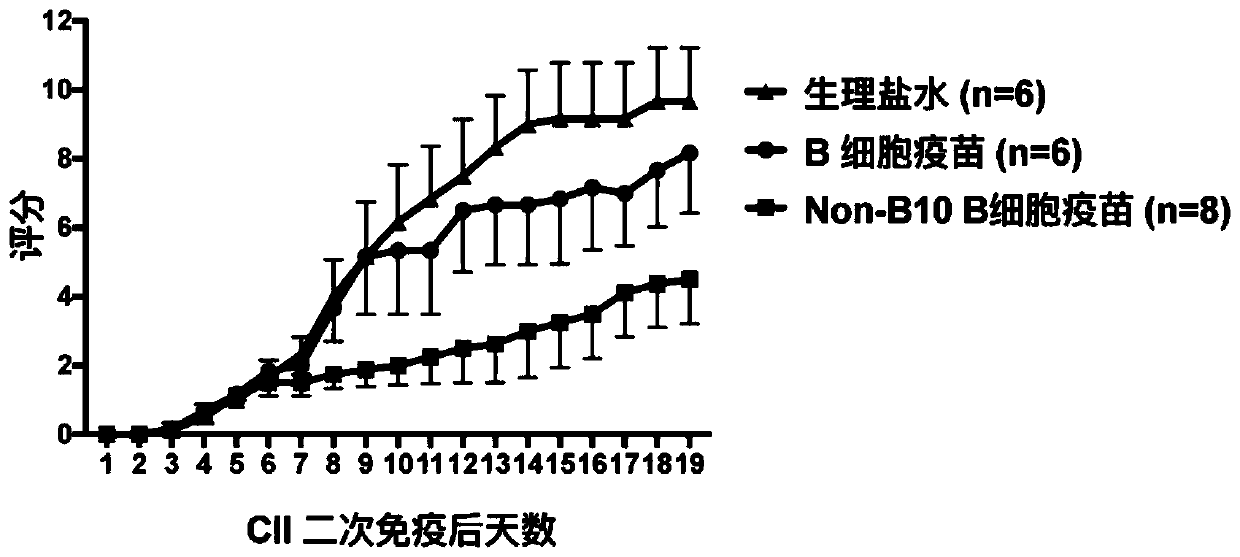
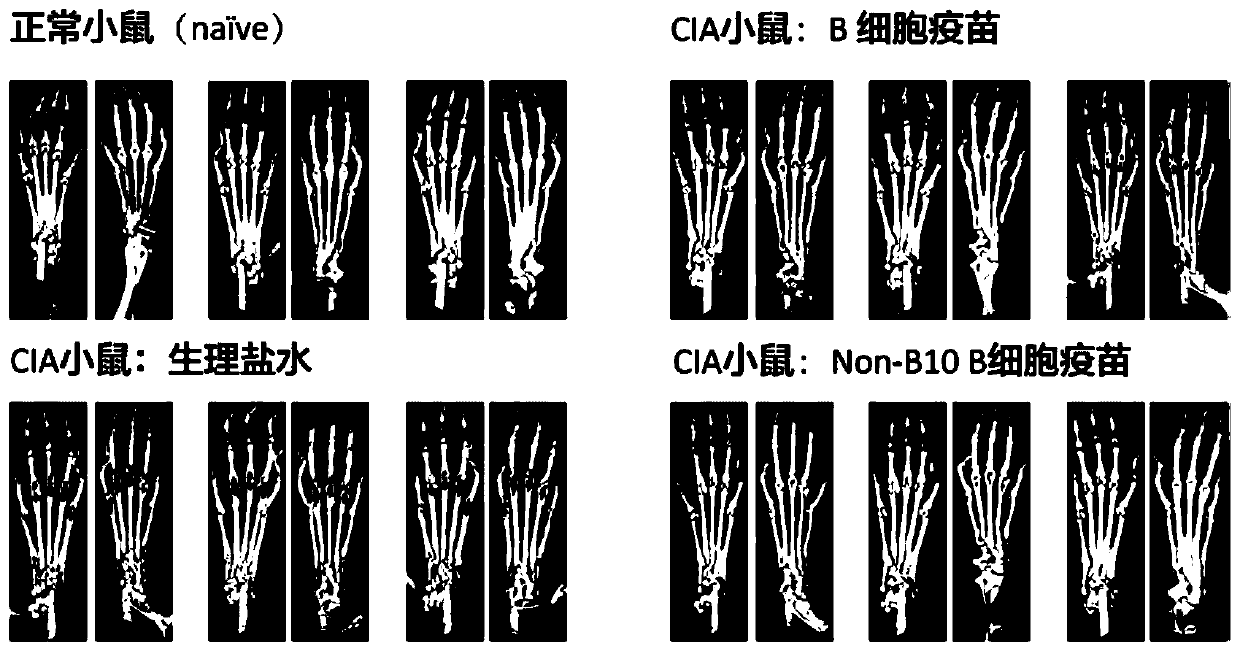
[0036] Modeling method: Bovine Type II Collagen (C II) was dissolved in 100 mmol / L glacial acetic acid overnight at 4°C, and mixed with complete Freund's adjuvant (CFA) at a volume ratio of 1:1. Fully emulsified. Inject subcutaneously at two points at the base of the tail of the mouse, each injecting an emulsion containing 100 μg of C II; on day 21 after the initial immunization, C II and incomplete Freund's adjuvant (IFA) were injected at a volume ratio of 1:1 Fully emulsify, and subcutaneously inject the emulsion containing 100 μg CII at the base of the tail of the mouse to comple...
Embodiment 2
[0052] The preparation of embodiment 2Non-B10 B cell vaccine
[0053] (1) According to the methods (1) to (6) in Example 1, spleen cell pellets were obtained.
[0054] (2) Add 300 μL of normal saline to resuspend the spleen cell pellet, add 5 μL of surface antibodies CD19-FITC, CD5-APC, and CD1d-PE in the dark, vortex and shake at room temperature for 30 minutes in the dark (vortex once every 15 minutes).
[0055] (3) Add 2 mL of normal saline solution, pipette and mix well, then centrifuge at 1800 rpm for 5 min and discard the supernatant.
[0056] (4) Add 1mL of sorting solution (PBS containing 2% FBS) to resuspend the cell pellet, and then run it on a flow cytometer (FACS AriaII sorter from BD Company), and sort out B10 cells (CD19 + CD5 + CD1d hi ) except all B cells (CD19 + CD5 - / + CD1d low ), that is, to obtain Non-B10 B cells. Each CIA mouse can be sorted 1.5-2.0*10 7 A Non-B10 B cell (reference: Aregulatory B cell subset with a unique CD1d hi CD5 + phenotype ...
Embodiment 3
[0059] The preparation of embodiment 3Plasma cell vaccine
[0060] (1) According to the methods (1) to (6) in Example 1, spleen cell pellets were obtained.
[0061] (2) Add 300 μL of normal saline to resuspend the spleen cell pellet, then add 5 μL of surface antibodies CD19-FITC and CD138-APC in the dark. Just add enough antibody to fully bind the cells to the antibody, and add 5-10 μL according to the number of cells in each tube.
[0062] (3) Using flow cytometry, the phenotype of the sorted cells is CD19 - / + CD138 + Plasma cells.
[0063] (4) Then adopt the same treatment mode as steps (9) to (14) in Example 1 to prepare Plasma cell vaccine. The concentration is 1×10 5 cell / 300μL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com