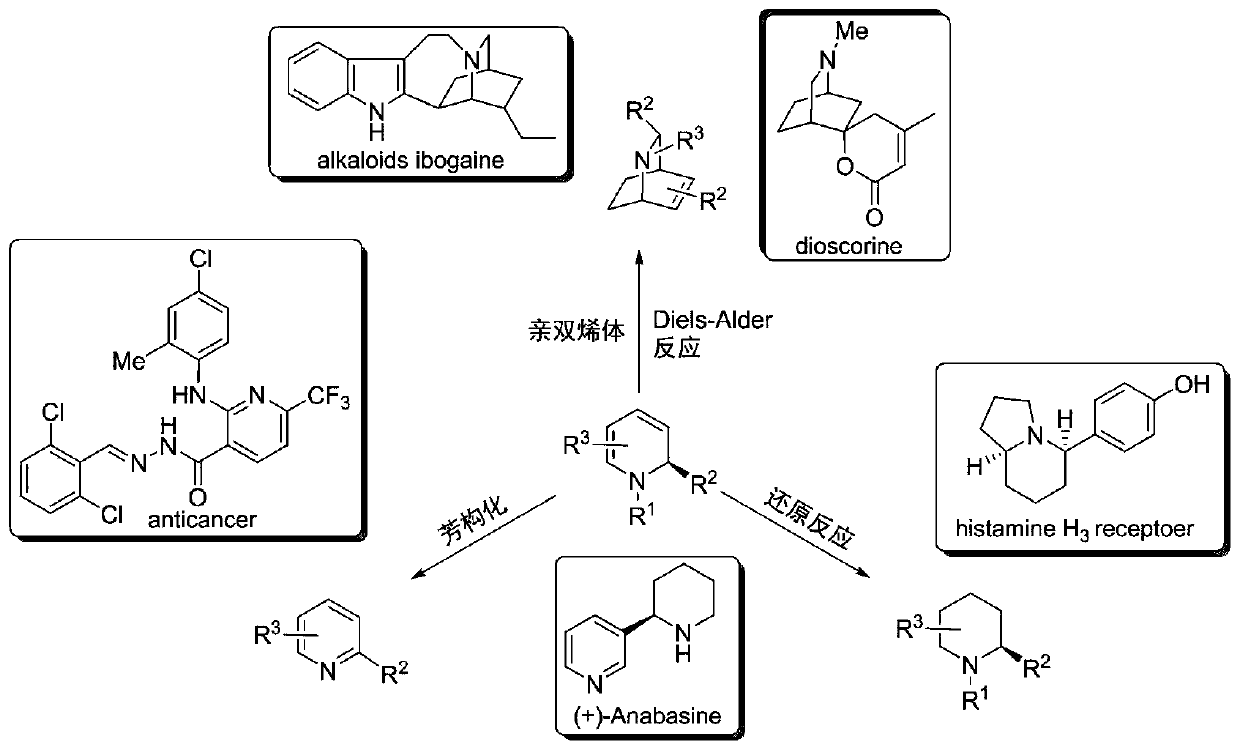
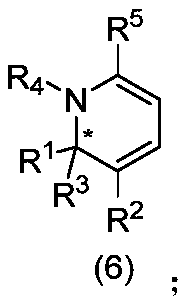
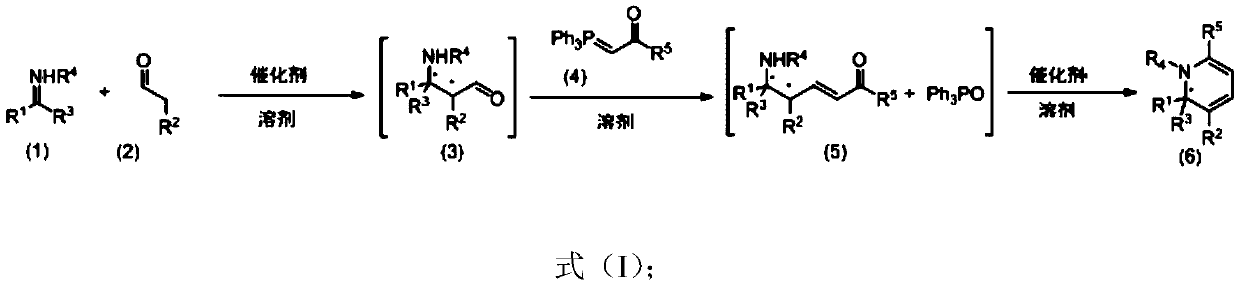
Chiral 1,2-dihydropyridine compound as well as preparation method and application thereof
A technology for dihydropyridine and aldehyde compounds, applied in the direction of organic chemistry, etc., can solve the problems of reduced applicability of synthetic methods, difficult synthesis, and poor reaction universality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162]
[0163]Under nitrogen protection, add imine 1a (410mg, 2.0mmol), propionaldehyde 2a (348mg, 6mmol) and dry redistilled acetonitrile (15mL) into a 25mL Schlenk tube, then stir at 0°C for 12h, a white solid Precipitation, acetonitrile and excess propionaldehyde 2a were removed by rotary evaporation. The white solid crude intermediate was transferred to a 10 mL Schlenk sealed tube, added dry redistilled ethyl acetate (6 mL), ylide 4a (636 mg, 2 mmol), and stirred at 80° C. for 12 h. Return to room temperature, add SiCl under nitrogen protection 4 (72μL, 0.6mmol), react at 40°C for 5h. The system was returned to room temperature, and the reaction solution was slowly added dropwise to 0°C saturated sodium bicarbonate solution (20 mL) to quench the reaction, extracted with ethyl acetate (3×15 mL), the organic phases were combined, and dried over anhydrous sodium sulfate. Column chromatography, eluent (petroleum ether / diethyl ether=20:1). Compound 6a was obtained as a c...
Embodiment 2
[0165]
[0166] Under nitrogen protection, add imine 1a (410mg, 2.0mmol), propionaldehyde 2a (348mg, 6mmol) and dry redistilled acetonitrile (15mL) into a 25mL Schlenk tube, then stir at 0°C for 12h, a white solid Precipitation, acetonitrile and excess propionaldehyde 2a were removed by rotary evaporation. The white solid crude intermediate was transferred to a 10 mL Schlenk sealed tube, dry redistilled dichloromethane (6 mL) and ylide 4b (664 mg, 2 mmol) were added, and stirred at 80° C. for 12 h. Return to room temperature, add SiCl under nitrogen protection 4 (48μL, 0.4mmol), react at 40°C for 8h. The system was returned to room temperature, and the reaction solution was slowly added dropwise to 0°C saturated sodium bicarbonate solution (20 mL) to quench the reaction, extracted with ethyl acetate (3×15 mL), the organic phases were combined, and dried over anhydrous sodium sulfate. Column chromatography, eluent (petroleum ether / diethyl ether=20:1). The colorless oily c...
Embodiment 3
[0168]
[0169] Under nitrogen protection, add imine 1a (422mg, 2.0mmol), propionaldehyde 2a (348mg, 6mmol) and dry redistilled acetonitrile (15mL) into a 25mL Schlenk tube, then stir at 0°C for 12h, a white solid Precipitation, acetonitrile and excess propionaldehyde 2a were removed by rotary evaporation. The white solid crude intermediate was transferred to a 10 mL Schlenk-sealed tube, added dry redistilled ethyl acetate (6 mL), ylide 4a (636 mg, 2 mmol), and stirred at 90° C. for 12 h. Return to room temperature, add SiCl under nitrogen protection 4 (72μL, 0.6mmol), react at 30°C for 5h. The system was returned to room temperature, and the reaction solution was slowly added dropwise to 0°C saturated sodium bicarbonate solution (20 mL) to quench the reaction, extracted with ethyl acetate (3×15 mL), the organic phases were combined, and dried over anhydrous sodium sulfate. Column chromatography, eluent (petroleum ether / diethyl ether=20:1). The colorless oily compound 6c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com