Preparation method of targeted drug AZD3759 intermediate
A technology of AZD3759 and intermediates, which is applied in the field of preparation of targeted drug AZD3759 intermediates, can solve the problems of highly toxic and unsafe phosphorus oxychloride, complex synthesis routes, expensive palladium carbon and the like, and achieves easy industrial production and process steps. Simple, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Preparation of 2-nitro-4-methoxy-5-hydroxybenzoic acid
[0045]
[0046]Dissolve 118g of sodium hydroxide in 400g of water, add 118g of 6-nitroveratronic acid, heat up to 100°C, keep warm for 6 hours, pour the system into 1160g of dilute hydrochloric acid, pH<2, cool down to room temperature, filter, and wash with water , and dried to obtain 108 g of a yellow solid, which is 2-nitro-4-methoxy-5-hydroxybenzoic acid, with a molar yield of 97.6% and a purity of 99%.
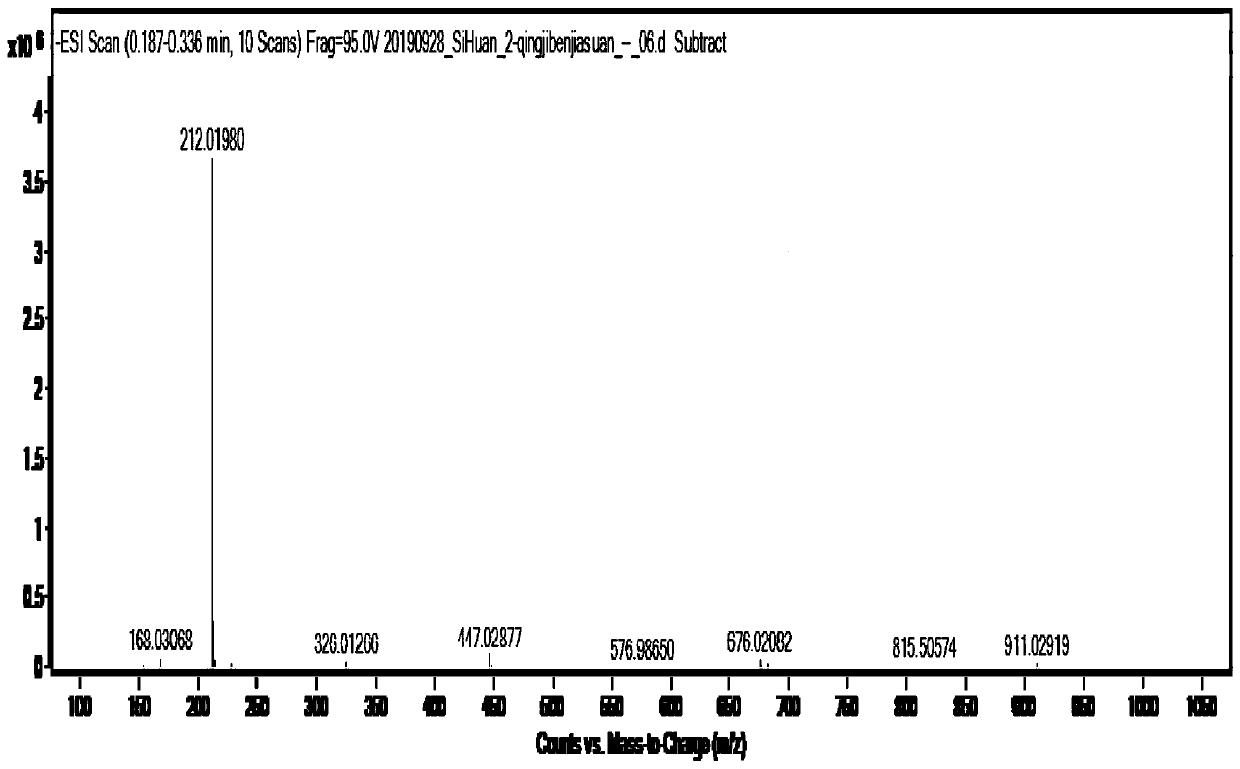
[0047] ESI-MS: m / z([M-H] - ) is 212.0, such as figure 1 shown.
Embodiment 2
[0048] Embodiment 2: Preparation of 2-amino-4-methoxy-5-hydroxybenzoic acid
[0049]
[0050] Add 100g of 2-nitro-4-methoxy-5-hydroxybenzoic acid to 500g of methanol, add 20g of a mixture of ferric chloride hexahydrate: active carbon=1:10 in mass ratio, rise to reflux, and add 40% dropwise Hydrazine hydrate 115g, keep reflux for 4h after dripping, after the reaction is completed, drop to room temperature, filter to remove the catalyst, and concentrate the solution to dryness to obtain 80g of off-white solid, which is 2-amino-4-methoxyl-5-hydroxybenzoic acid, molar yield The yield is 93.1%, and the purity is 99%.
Embodiment 3
[0051] Embodiment 3: the preparation of 4,6-dihydroxy-7-methoxyquinazoline
[0052]
[0053] Add 80g of 2-amino-4-methoxy-5-hydroxybenzoic acid and 90g of methylamine acetate to 450g of ethylene glycol methyl ether, raise the temperature to 110°C, keep it warm for 6h, after the reaction is complete, concentrate to dryness under reduced pressure, add 450g Water, 50g of ammonia water, stirred at room temperature for 2h, filtered, and dried at 50°C for 6h to obtain 71g of brown solid, namely 4,6-dihydroxy-7-methoxyquinazoline, with a molar yield of 84.6% and a purity of 99.2%.
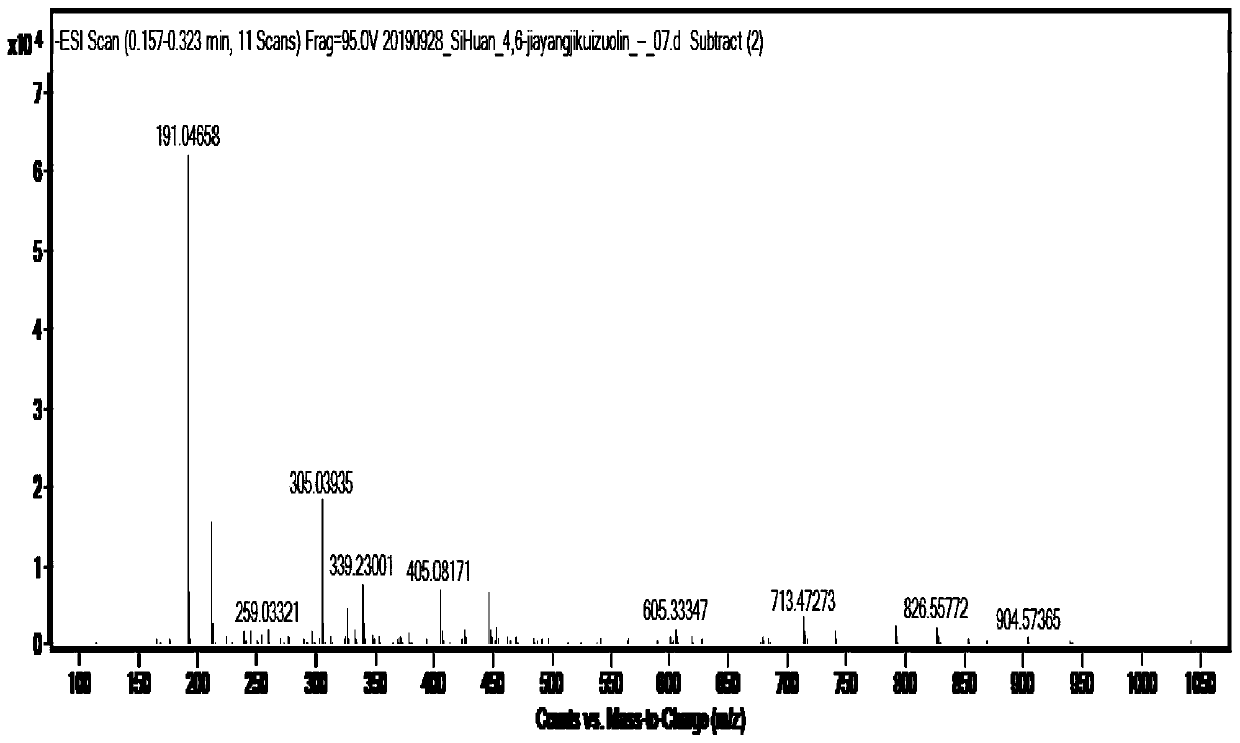
[0054] ESI-MS: m / z([M-H]-) is 191.0, such as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com