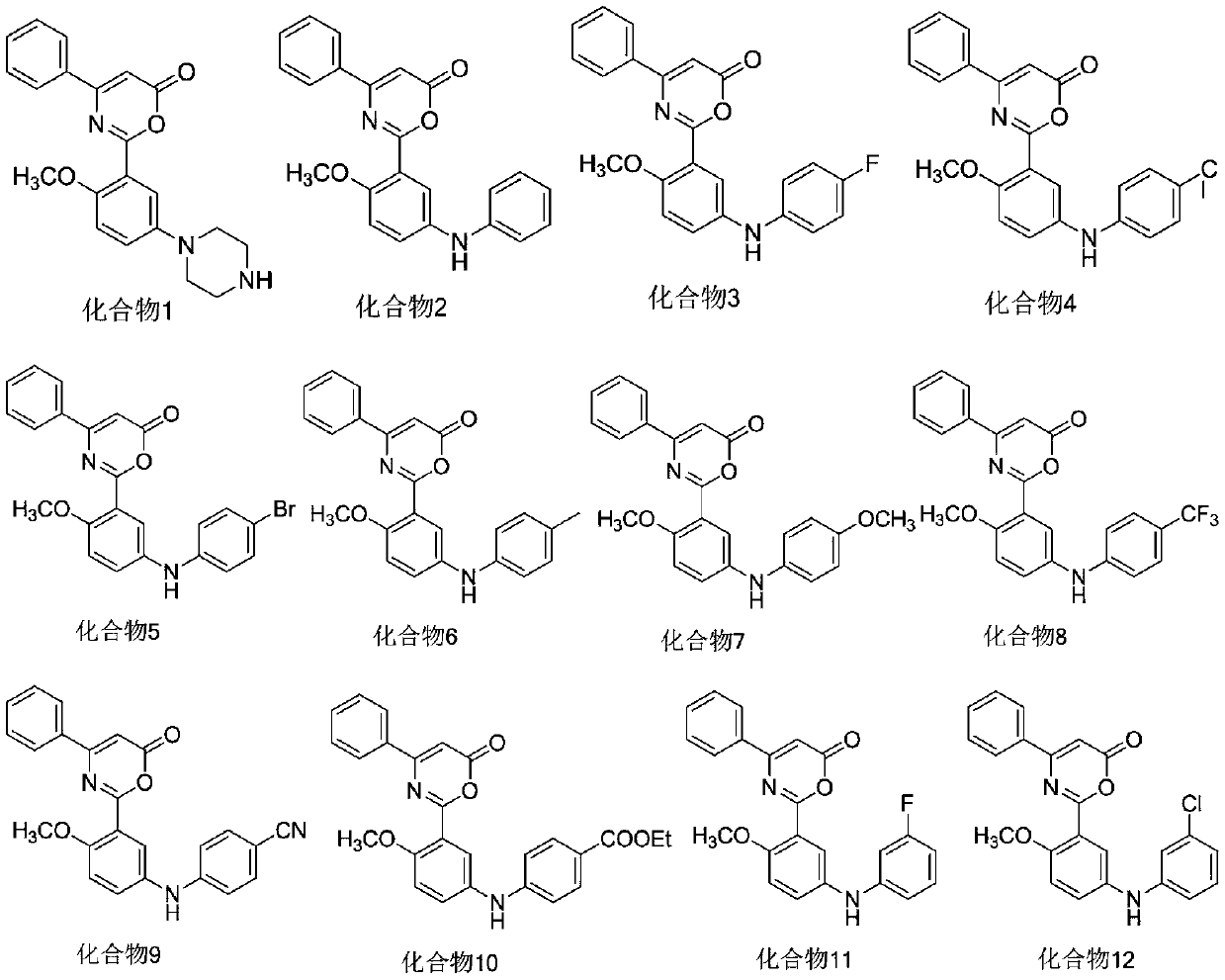
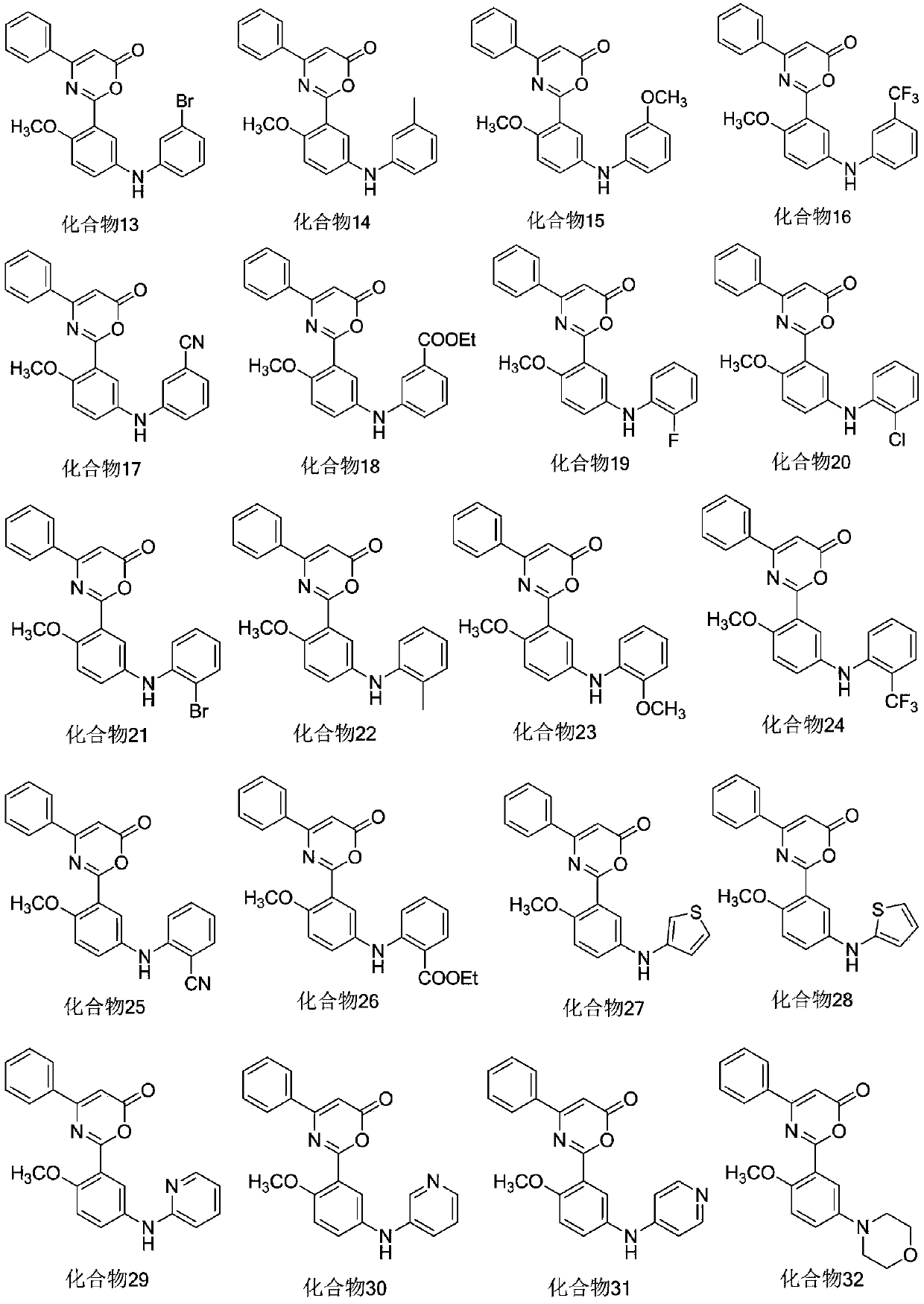
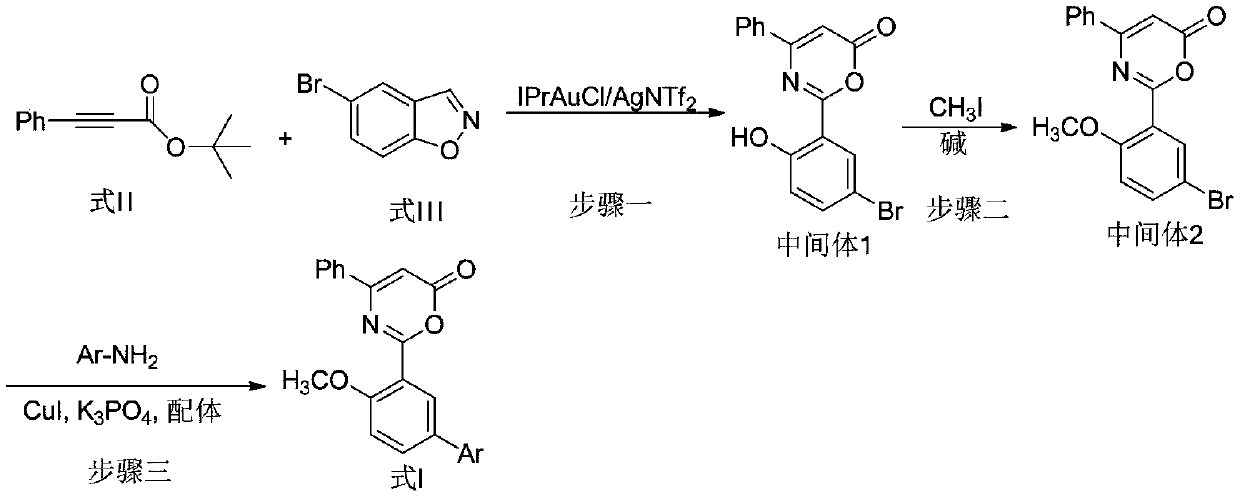
6H-1,3-oxazoline-6-one compound and uses in drugs for treating attention deficit hyperactivity disorder
A ketone compound, 6H-1 technology, applied in the field of medicine, can solve the problems of adolescent height and weight growth retardation, atomoxetine hydrochloride self-injury, adverse effects of blood pressure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the synthesis of intermediate 1
[0016]
[0017] Add IPrAuCl (0.050mmoL), AgNTf 2 (0.050mmoL) and dry DCE (1.0mL). After the mixture was stirred at room temperature for 10 min, a dry DCE solution (5 mL) of tert-butyl 3-phenylpropionate (1.0 mmol) and 5-bromo-1,2-benzisoxazole (1.2 mmol) was added dropwise. After the dropwise addition was completed, the temperature of the reaction solution was slowly raised to 80° C. and heated for 2 d. After the completion of the reaction monitored by TLC, it was cooled to room temperature. The reaction solution was filtered with celite, and the filter cake was rinsed with dichloromethane. The combined filtrate was concentrated and purified by column chromatography (PE / EA=3 / 1) to obtain intermediate 1. Yellow solid, yield 87%. 1 H-NMR (400MHz, CDCl 3 ):δ12.45(s,1H),8.13(s,1H),7.80(d,J=7.6Hz,2H),7.59-7.52(m,4H),6.95(d,J=8.8Hz,1H) ,6.53(s,1H). ESI-MS(m / z)=345.2[M+H] + .
Embodiment 2
[0018] Embodiment 2: the synthesis of intermediate 2
[0019]
[0020] Intermediate 1 (1.0mmoL) and acetone (8mL) were added to the round bottom flask, and potassium carbonate (5.0mmoL) was added after stirring to dissolve. After stirring at room temperature for 10 min, iodomethane (1.3 mmol) was added. The reaction solution was heated and stirred at reflux for 10 h. TLC monitored the completion of the reaction and added saturated brine and dichloromethane for liquid separation and extraction. The combined organic phases were dried over anhydrous sodium sulfate and filtered, the filtrate was concentrated by rotary evaporation, and the residue was purified by column chromatography (PE / EA=5 / 1) to obtain intermediate 2. Yellow solid, yield 92%. 1 H-NMR (400MHz, CDCl 3 ):δ8.15(s,1H),7.82(d,J=7.6Hz,2H),7.60-7.51(m,4H),6.94(d,J=8.8Hz,1H),6.54(s,1H) ,3.81(s,3H). ESI-MS(m / z)=359.2[M+H] + .
Embodiment 3
[0021] Embodiment 3: the synthesis of compound 1
[0022]
[0023] After sealing the tube and blowing nitrogen for 5min, add intermediate 2 (4.0mmoL), piperazine (4.8mmoL), CuI (0.02mmoL), K 3 PO 4 (8.0mmoL), N,N′-bis(furan-2-methyl)oxalamide (BFMO, 0.02mmoL) and ethanol (4mL), blow with nitrogen for 5min and immediately tighten the cork. The mixture was heated at 80° C. for 10 h, and cooled to room temperature after the reaction was complete as monitored by TLC. The reaction solution was filtered with celite, and the filter cake was rinsed with dichloromethane. The combined filtrate was concentrated and purified by column chromatography (PE / EA=2 / 1) to obtain compound 1. Yellow solid, yield 65%. 1 H-NMR (400MHz, CDCl 3 ):δ8.02(s,1H),7.69(d,J=7.6Hz,2H),7.55-7.48(m,4H),6.60(d,J=8.8Hz,1H),6.37(s,1H) ,3.62(s,3H),3.41-3.38(m,4H),2.98-2.88(m,4H). ESI-MS(m / z)=364.4[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com