Preparation method of beta-lactamase inhibitor drug avibactam sodium intermediate
A technology of avibactam sodium and lactamase, which is applied in the field of medicinal chemistry, can solve the problems of affecting the yield of target product compounds, reducing the yield of compound III, poor stability of intermediate IIa, etc., and achieving safe, rapid and continuous reactions, Avoid excessive accumulation, the effect of less amount of chemicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
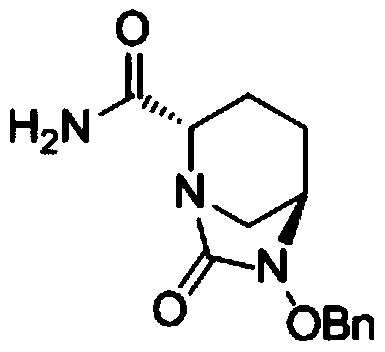
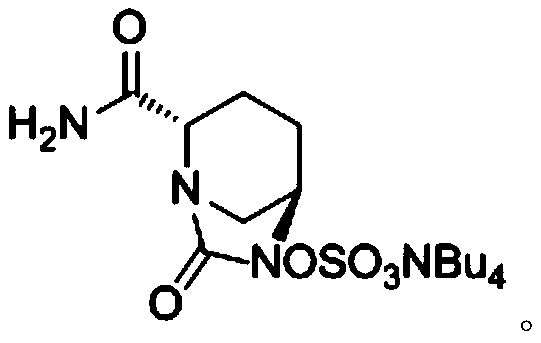
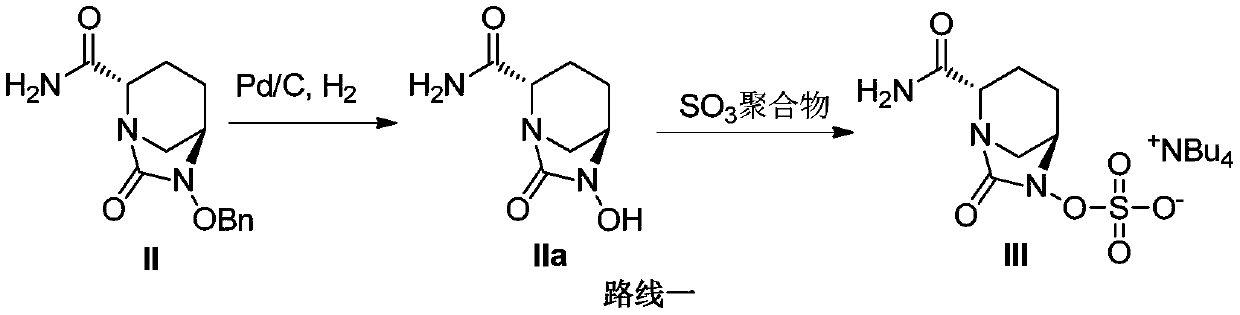
[0061] ({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-octyl-6- Base] Oxygen} Sulfonyl) Tetrabutylammonium Salt (Compound III) Preparation
[0062] (1) Add isopropanol (500mL) and purified water (500mL) into the reaction flask, add compound II (100.0g), sulfur trioxide trimethylamine copolymer (100.0g), triethylamine (30g) and palladium carbon (10.0g), stirred evenly at room temperature for later use.
[0063] (2) Using Corning G1 reactor, the hydrogen pressure is set to 0.5MPa, the hydrogen flow rate is 0.5NL / min (22.3mmol / min), and the feed rate is 40g / min (according to intermediate II, it is 12.9mmol / min) , the reaction temperature was set at 35°C, and the equipment was operated stably.
[0064] (3) Turn on the feed pump, inject the reaction material into the reactor through the feed pump to start the reaction, and after about 1 min, the reaction liquid flows out from the reactor to the material collection tank.
[0065] (4) Filtrate and collect the fil...
Embodiment 2
[0068] ({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-octyl-6- Base] Oxygen} Sulfonyl) Tetrabutylammonium Salt (Compound III) Preparation
[0069] (1) Add acetonitrile (500mL) and purified water (500mL) into the reaction flask, add compound II (100.0g), sulfur trioxide trimethylamine copolymer (100.0g), triethylamine (30g) and palladium carbon (10.0 g), stirring evenly at room temperature for subsequent use.
[0070] (2) Using Corning G1 reactor, the hydrogen pressure is set to 0.5MPa, the hydrogen flow rate is 0.5NL / min (22.3mmol / min), and the feed rate is 40g / min (according to intermediate II, it is 12.9mmol / min) , the reaction temperature was set at 35°C, and the equipment was operated stably.
[0071] (3) Turn on the feed pump, inject the reaction material into the reactor through the feed pump to start the reaction, and after about 1 min, the reaction liquid flows out from the reactor to the material collection tank.
[0072] (4) Filtrate and collect...
Embodiment 3
[0075] ({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-octyl-6- Base] Oxygen} Sulfonyl) Tetrabutylammonium Salt (Compound III) Preparation
[0076] (1) Add isopropanol (10L) and purified water (10L) into the reaction flask, add compound II (2kg), sulfur trioxide trimethylamine copolymer (2kg), triethylamine (600g) and palladium carbon (200.0 g), stirring evenly at room temperature for subsequent use.
[0077] (2) Using Corning G4 reactor, the hydrogen pressure is set to 0.5MPa, the hydrogen flow rate is 15NL / min (0.67mol / min), and the feed rate is 1.2kg / min (0.39mol / min according to intermediate II) , the reaction temperature was set at 35°C, and the equipment was operated stably.
[0078] (3) Turn on the feed pump, inject the reaction material into the reactor through the feed pump to start the reaction, and after about 1 min, the reaction liquid flows out from the reactor to the material collection tank.
[0079] (4) Filtrate and collect the filtrate, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com