Synthetic method of rhodiola rosea active ingredient rosavin or rosarin
A technology of active ingredients and synthesis methods, applied in the field of compound synthesis, can solve the problems of no application prospect, unsatisfactory overall yield, heavy metal residues, etc., and achieve the solution of unstable yield, good industrialization prospects, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
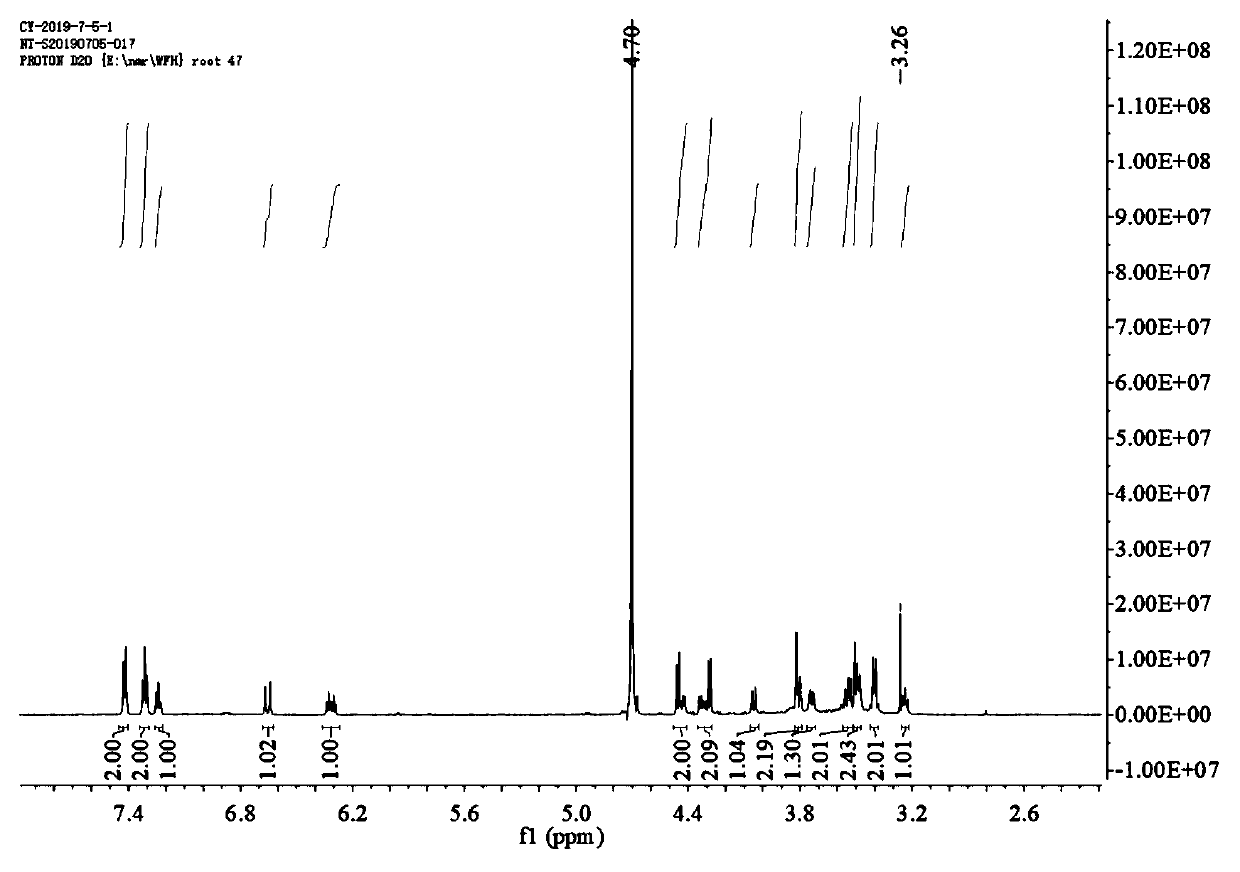
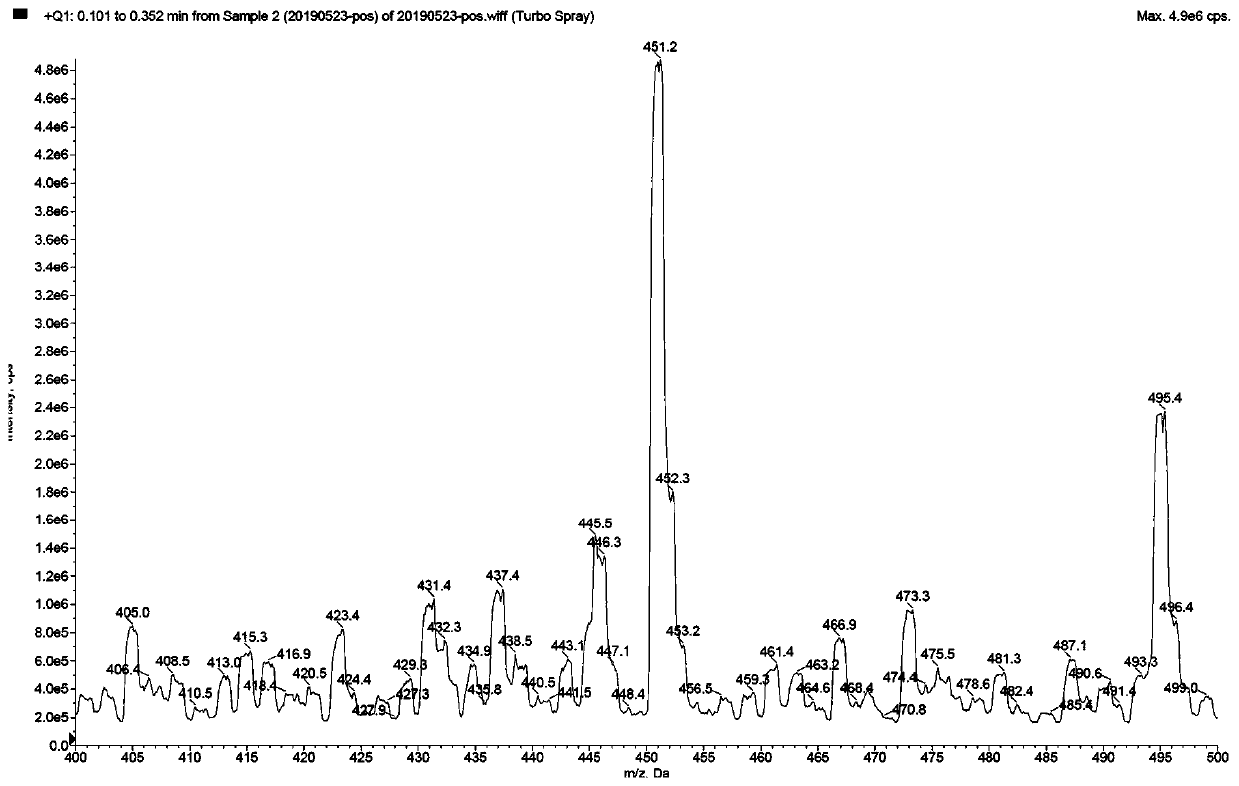
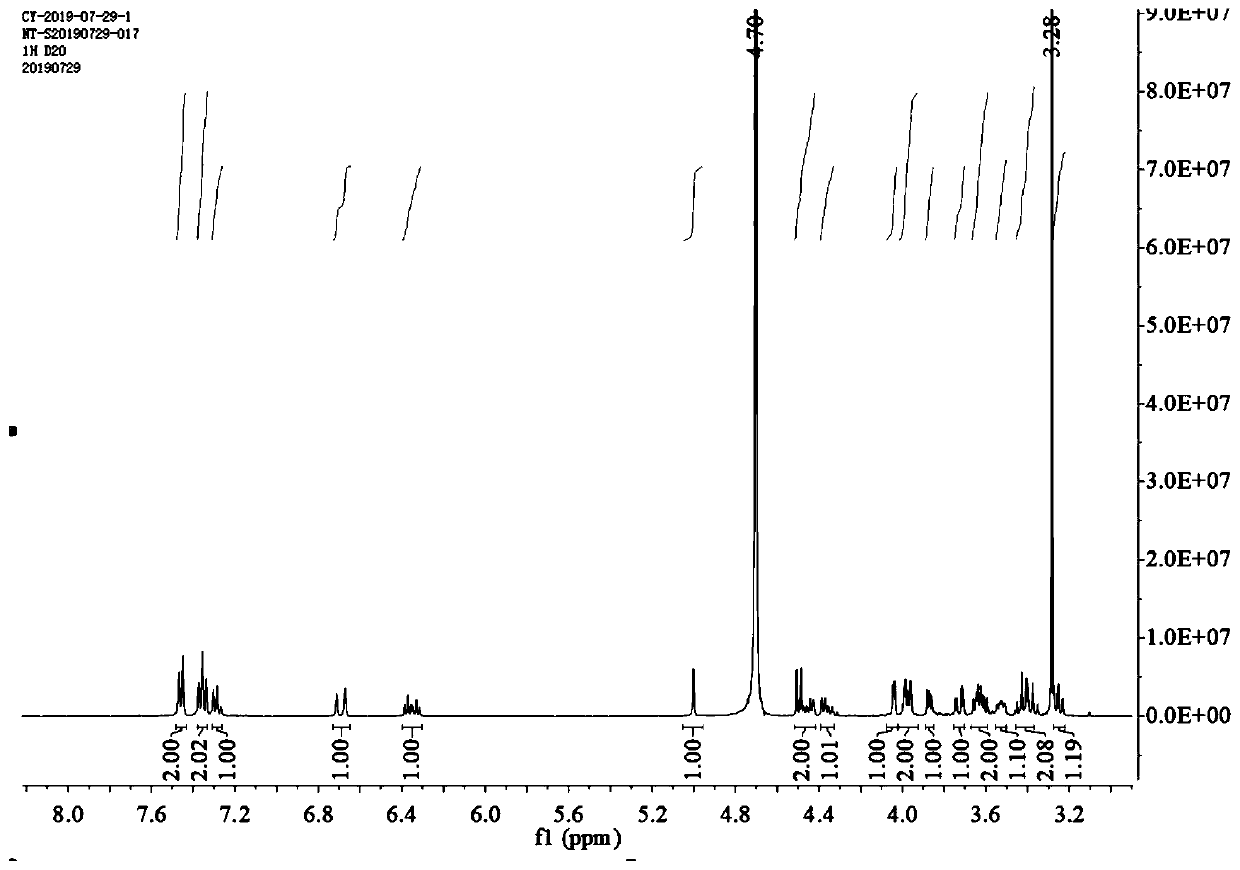
Image
Examples
Embodiment 1
[0038]
[0039]Stir 50g of L-arabinose in 800mL of pyridine, gradually add 270g of acetic anhydride, the system gradually turns light yellow and transparent, then stir and react overnight at room temperature, add 100mL of methanol to quench the reaction after the reaction, and then spin off the system solvent , the remaining oil was dissolved with 500ml of dichloromethane, washed successively with 200mL of 1M hydrochloric acid and 200mL of saturated sodium bicarbonate, then the organic phase was collected and dried over anhydrous sodium sulfate. After drying, the solvent was spun off to obtain 126g of yellow oil, namely Dissolve fully acetylated arabinose in 300mL of dichloromethane, add 42g of thiophenol and 55g of 47% boron trifluoride ether solution in turn, and stir the system at room temperature overnight. After the reaction, add 50g of triethylamine After the reaction was quenched, the system solvent was spun off, and the remaining oil was subjected to rapid silica gel...
Embodiment 2
[0042]
[0043] Dissolve 50g of fully acetylated arabinose in 200mL of dichloromethane, then add 32g of iodine and 21g of hexamethyldisilazane (HMDS), stir at room temperature for 3 hours, and add 13g of dimethyl disulfide after the conversion is complete. The system was stirred and reacted overnight at room temperature. After the reaction was completed, the system was concentrated and purified on a silica gel column to obtain 42 g of a light yellow oily substance, sugar donor A2, with a yield of 87%.
[0044] 1 H NMR (400MHz, CDCl 3 )δ5.56(d,J=2.0Hz,1H),5.31(s,1H),5.25–5.30(m,1H),5.10–5.15(m,1H),4.53–4.37(m,1H),4.15 –4.33(m,1H),2.15(s,3H),1.90–2.10(m,9H).
Embodiment 3
[0046]
[0047] Dissolve 50g of fully acetylated arabinose in 100mL of 37% hydrogen bromide acetic acid solution, stir the system at room temperature overnight, then spin off the solvent, dissolve the remaining oil in 500mL of dichloromethane, and then add saturated sodium bicarbonate The pH of the system was adjusted to neutral with the solution, and then the organic phase was collected and dried over anhydrous sodium sulfate. After drying, the solvent was spun off to obtain 38 g of a slightly yellow oily substance, ie sugar donor A3, with a yield of 71%.
[0048] 1 H NMR (400MHz, CDCl 3 )δ6.62(m,1H),5.38–5.41(m,1H),4.96–5.05(m,1H),4.05–4.15(m,2H),3.53–3.90(m,1H),2.01–2.15( m, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com