Structure of human recombinant methionine sulfoxide reductase A (MsrA) protein, and construction method of structure
A technology of methionine sulfoxide and construction method, which is applied in the field of structure and construction of human recombinant methionine sulfoxide reductase A protein, to improve the anti-As effect of HDL
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1 of the present invention provides the structure of a secreted human recombinant methionine sulfoxide reductase A protein, including:
[0094] The structure contains a segment of human apoE signal peptide and EpK peptide, as shown in SEQ ID NO.4:
[0095] Met Lys Val Leu Trp AlaAla Leu Leu Val Thr Phe LeuAla Gly Cys GlnAlaHis His His His His His Leu Arg Lys LeuArg Lys Arg Leu Leu Arg Lys Lys LysLys Lys Lys Lys Gln Val Ala Glu Val Arg Ala Lys Leu Glu Glu Gln Ala Gln Gln IleArg Leu Gln Ala Glu
[0096] Wherein, the complete structure of the EpK peptide is expressed as shown in SEQ ID NO.2:
[0097] LeuArg Lys LeuArg Lys Arg Leu LeuArg Lys Lys Lys Lys Lys Lys GlnValAla GluValArgAla Lys Leu Glu GlnAla Gln Gln IleArg Leu GlnAla Glu;
[0098] The structure of MsrA is a 213-amino acid chain without signal peptide, which is connected to form a complete EpK-MsrA fusion protein polypeptide chain through the coding amino acids of BamHI restriction endonuclease recogniti...
Embodiment 2
[0121] The embodiment of the present invention aims at the construction structure proposed in the embodiment 1, and elaborates on how to demonstrate its effect through experiments. Specifically, the experimental research is conducted on the in vivo function of the recombinant Epk-MsrA fusion protein. Including the following process:
[0122] Process 1. Packaging of recombinant lentiviral particles containing human Epk-MsrA
[0123] The packaging of the recombinant lentiviral particles containing human Epk-MsrA comprises the following steps:
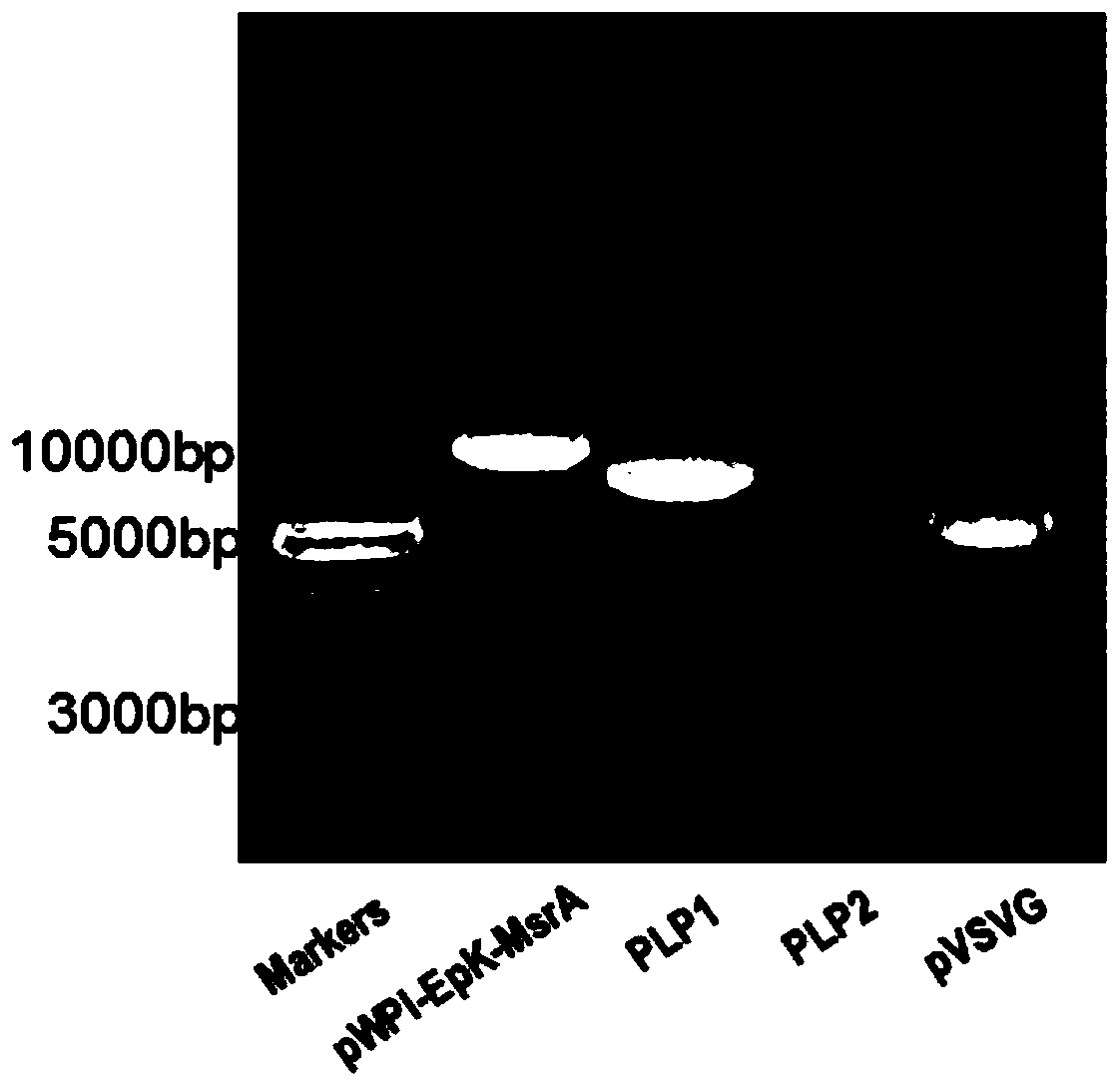
[0124]Step 1. Extraction and identification of lentiviral vectors and packaging plasmids: Inoculate the pWPI-Epk-MsrA recombinant plasmid (pWPI-GFP as a control) and the DH5α strains of the three lentiviral packaging plasmids PLP1, PLP2, and pVSVG, respectively, into cells containing In 250ml of liquid LB medium with 100μg / ml ampicillin, shake at 37°C and 200-300rpm for 12-16h; the next day, centrifuge the bacterial culture solution a...
Embodiment 3
[0147] The embodiment of the present invention provides the construction of a recombinant human Epk-MsrA fusion protein expressed by prokaryotic cells, including:
[0148] The recombinant prokaryotic expression vector pET28a-EpK-MsrA was constructed by genetic engineering technology, and the recombinant EpK-MsrA fusion protein expressed in Escherichia coli was purified by NTA-Ni column affinity chromatography to obtain the purified EpK-MsrA fusion protein.
[0149] In the embodiment of the present invention, because the pET8a prokaryotic expression vector containing the His-tag and the Escherichia coli host bacteria are used, the signal peptide sequence of EpK and the 6-His sequence in the middle of the structure are no longer designed (as presented in Example 1) ), a typical basic structure of EpK-MsrA described in the embodiments of the present invention, as shown in SEQ ID NO.5:
[0150] Met Cys Leu Arg Lys Leu Arg Lys Arg Leu Leu Arg Lys Lys Lys Lys Lys Lys Gln Val Ala Glu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com