Four-electron homogeneous reducing agent, preparation method and applications thereof
A reducing agent and electron technology, applied in the preparation of reducing agent, the field of homogeneous reducing agent, can solve problems such as restricting the research of chemical properties and uses, and achieve the effects of good chemical stability, environmental friendliness and safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of 2,3,4,5,2',3',4',5'-octaphenylspirocyclic silole tetralithium salt
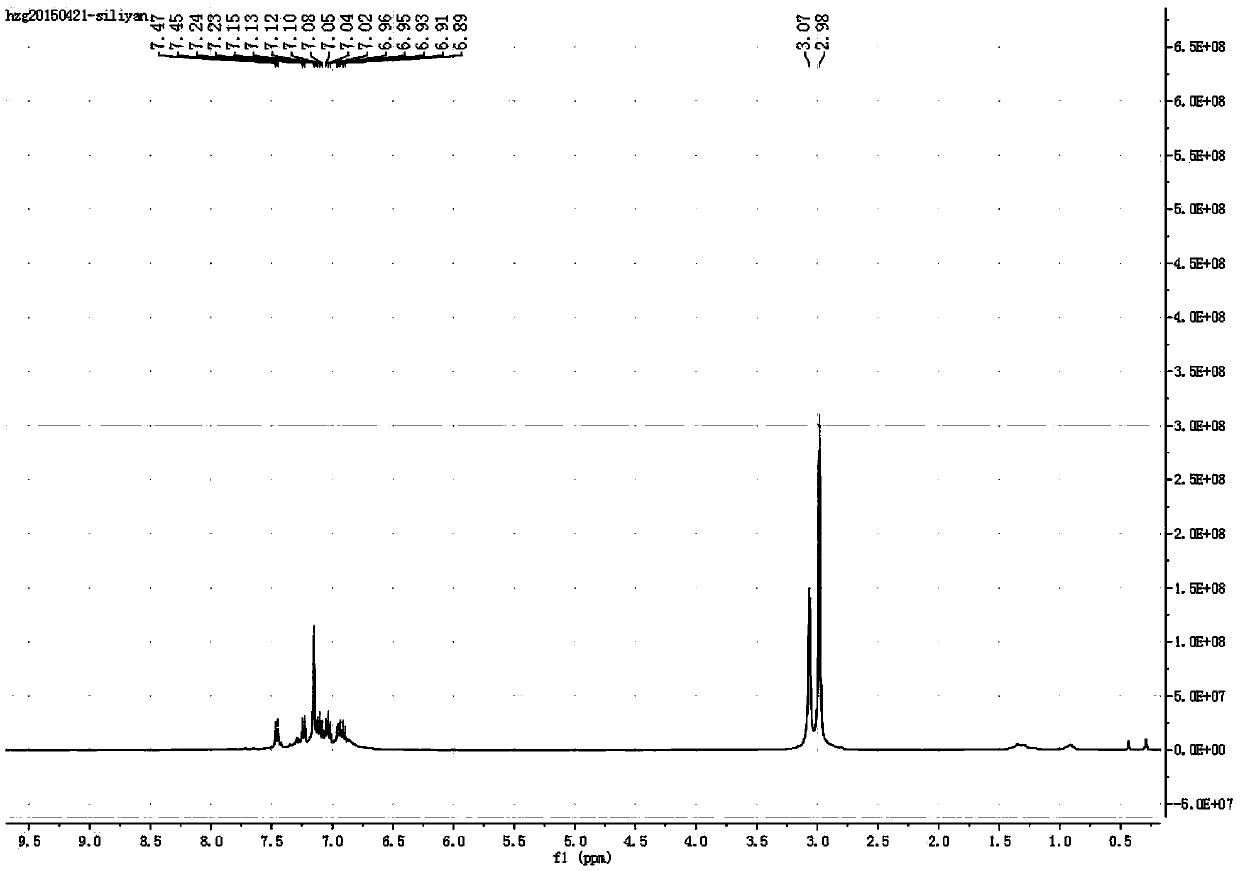
[0053] Under the protection of nitrogen, metal lithium (0.30 g, 44.0 mmol) was added to 2,3,4,5,2',3',4',5'-octamol dissolved in 50 mL of ethylene glycol dimethyl ether at room temperature Phenylspirosilole (7.4 g, 10.0 mmol). React for 2h, concentrate the solution to 10mL, and place it in a refrigerator at -40°C for crystallization to obtain orange-red solid 2,3,4,5,2',3',4',5'-octaphenylspirocyclic silole Tetralithium salt 11.3g, yield 95%.
[0054] The obtained product was subjected to H NMR spectroscopy ( 1 H NMR C 6 D. 6 )like figure 1 . It can be seen from the figure that δ=6.87~7.47 ppm is the peak of the eight phenyl groups of spirocyclic silole, and δ=2.98 and δ=3.07ppm are the peaks of methylene and methyl groups on ethylene glycol dimethyl ether , Compared with the peaks of free ethylene glycol dimethyl ether (δ=3.12 and δ=3.33ppm), significant changes have ta...
Embodiment 2
[0062] The reaction of embodiment 2 spirocyclic silole tetralithium salts and diquinones
[0063] The preparation process of 2,3,4,5,2',3',4',5'-octaphenylspirosilole tetralithium salt is the same as in Example 1.
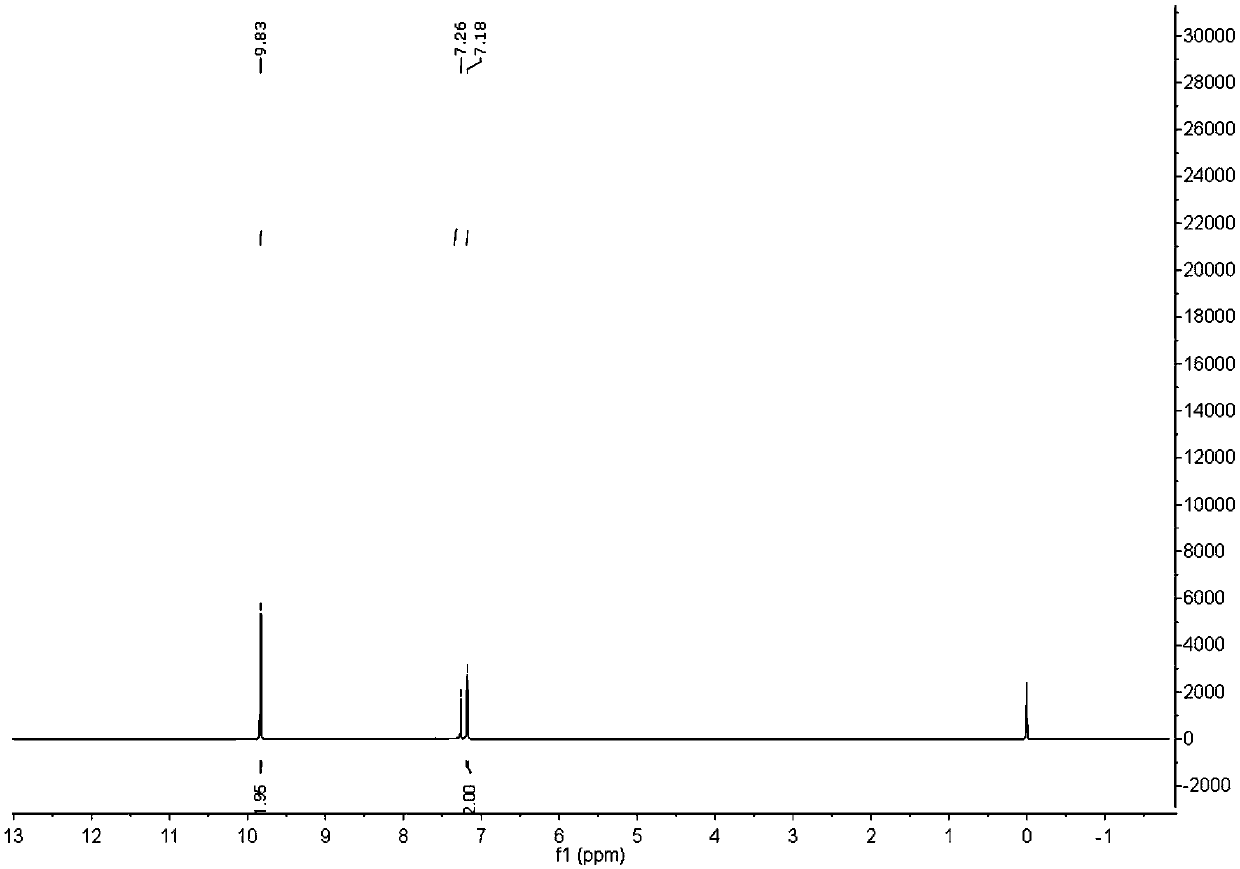
[0064] At room temperature, a solution of diquinone (0.027 g, 0.25 mmol) dissolved in 10 mL of tetrahydrofuran was added dropwise to the octaphenylspirocyclic silole tetralithium salt (0.80 g, 0.50 mmol) obtained in Example 1, and the color of the system gradually changed to colorless. Drain and make the crude product NMR shows (such as image 3 ) generates hydroquinone anion (peak at σ=9.83ppm), and the spirocyclic silole tetralithium salt is completely transformed into a spirocyclic silole compound.
[0065] It shows that the tetralithium salt of spirocyclic silole can effectively reduce diquinone as a homogeneous four-electron reducing agent, showing the properties of a homogeneous reducing agent.
Embodiment 3
[0072] The reaction of embodiment 3 spirocyclic silole tetralithium salts and methyl iodide
[0073] The preparation process of 2,3,4,5,2',3',4',5'-octaphenylspirosilole tetralithium salt is the same as in Example 1.
[0074] At room temperature, a solution of methyl iodide (0.07 g, 0.5 mmol) dissolved in 5 mL of tetrahydrofuran was added dropwise to the obtained spirocyclic silole tetralithium salt (0.40 g, 0.25 mmol, the product of Example 1) obtained in Example 1, and the system The color gradually becomes colorless. Draining and doing the NMR of the crude product showed that the reduction product methyllithium (MeLi) was generated, and the spirocyclic silole tetralithium salt was completely converted into the spirocyclic silole compound.
[0075] It shows that the tetralithium salt of spirocyclic silole can effectively reduce methyl iodide as a homogeneous four-electron reducing agent, showing the properties of a homogeneous reducing agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com