A kind of preparation and refining method of high-purity empagliflozin
A refining method and concentration technology, which is applied in the field of preparation and refining of high-purity empagliflozin, can solve problems such as increased production costs, harsh use conditions, and low yields, and achieve the effects of saving production costs and being conducive to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
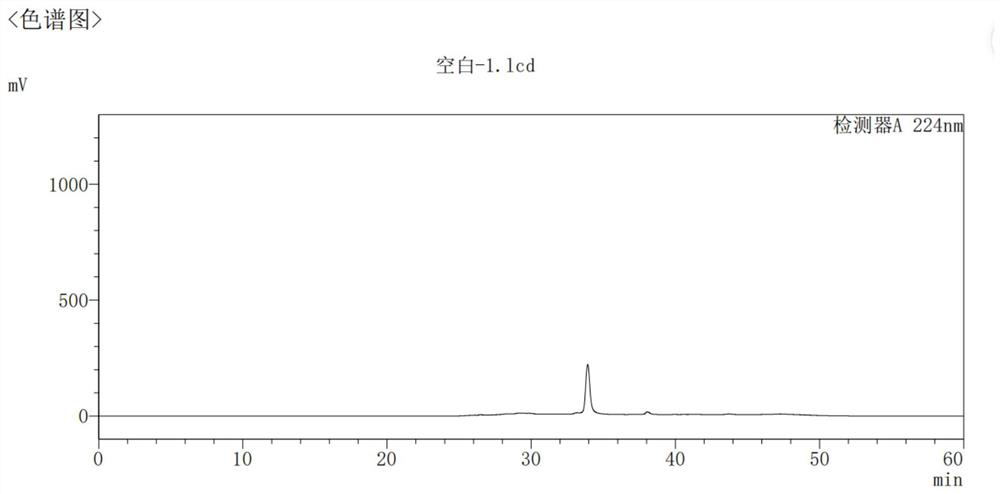
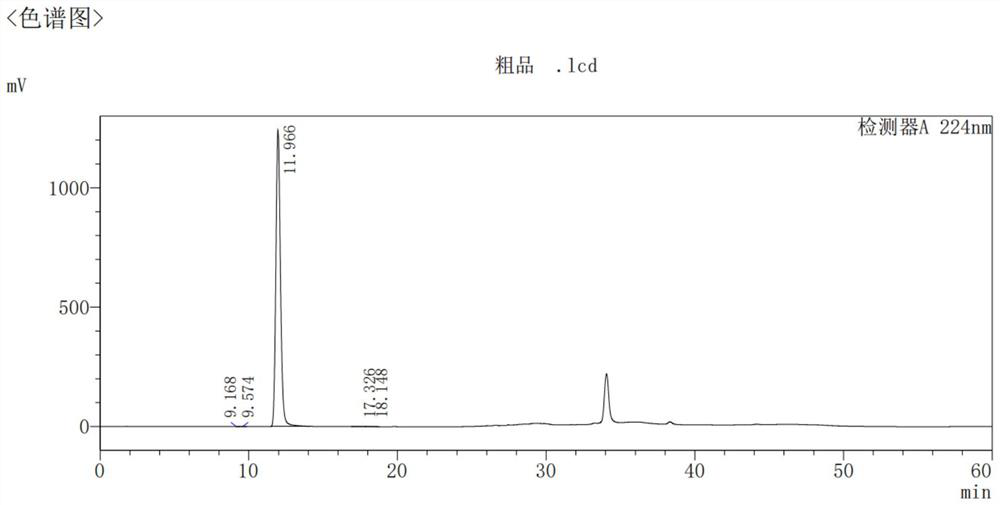
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of crude product
[0069] Add SM1 (2000g) and THF (3575ml) to a dry 20L four-necked bottle under nitrogen protection, install a tail gas absorption device, stir and dissolve completely at 20-25°C, cool the inner temperature to -16°C, and add (1.3N) dropwise Isopropylmagnesium chloride-lithium chloride THF solution 4410ml, control the internal temperature at -14±2°C during the dropwise addition, drop it in 1.5 hours, continue to stir and react for 0.5 hours, add 2251.5g SM2 dropwise between the temperature control -14±2°C, After 50 minutes of dropwise addition, continue to keep warm for 1 hour, and TLC detects that the reaction is basically complete (developing solvent: petroleum ether / ethyl acetate=3 / 1).
[0070] Control the internal temperature below 5°C and add 6,000ml of 10% citric acid aqueous solution dropwise. After 20 minutes, the dropwise addition is completed, and continue to stir for 5 minutes. Separate the upper organic phase, ex...
Embodiment 2
[0076] Embodiment 2: the refinement of embodiment 1 crude product
[0077] Refining: Add 200 g of the crude product into a 2 L single-necked bottle, add 400 mL of ethanol, and 800 mL of (1N) NaOH aqueous solution. Heat to reflux to dissolve the clear. Add 10 g of activated carbon, stir for 1 hour, and filter with suction. Heat (ethanol: water = 1:2) to wash the filter cake. The filtrate was heated to reflux and added to 6.4 L of (1N) NaOH aqueous solution at 80°C. After the dropwise addition was completed, the mixture was stirred at 15-20° C. for 16 hours. Suction filtration, the solid was washed with purified water and suction filtration until neutral. The wet product was air-dried at 50° C. for 15 hours to obtain 192.5 g of dry product. Yield: 96.25%. HPLC: 99.97%. (See Figure 4 )
Embodiment 3
[0078] Example 3: Comparison of the solvent effects of the crystallization of the crude product of Empagliflozin
[0079] Using the oil of Example 1 as a raw material to study the solvent effect of crystallization of the crude product, the oil: impurity 1: 5.038%, impurity 2: 2.375%, and total other impurities: 16.242%.
[0080] In this example, the system numbered 6 is Example 1.
[0081] Solvent system No. 1-5: Add the mixed solvent or solvent in the stated proportion to the oil at room temperature at one time, then stir at 10-15°C for 15 hours, and filter with suction to obtain the crude product.
[0082] Table 1: Comparison of impurities and purity after crystallization of crude products in different solvents:
[0083]
[0084] As can be seen from Table 1, the present invention selects different crude product crystallization solvents, oily matter: dichloromethane: 1N NaOH aqueous solution is 1:3:3 in dosage ratio, and temperature crystallizes under the condition of 10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com