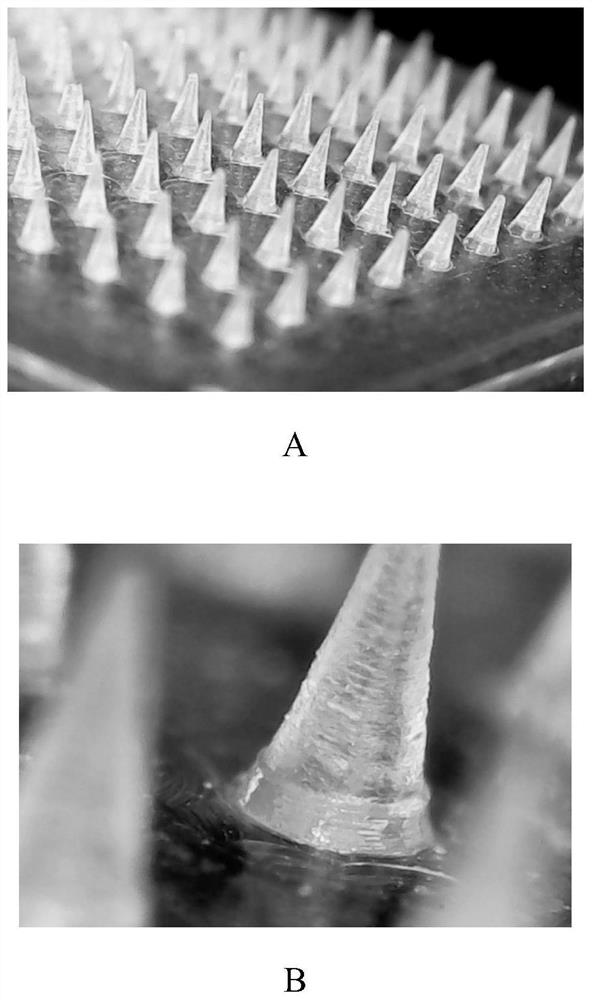
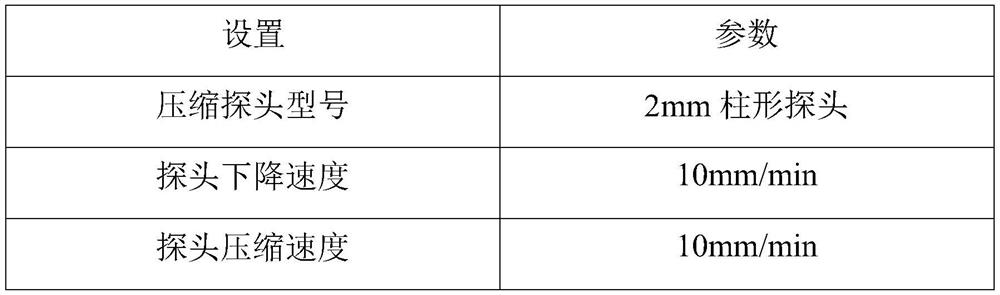
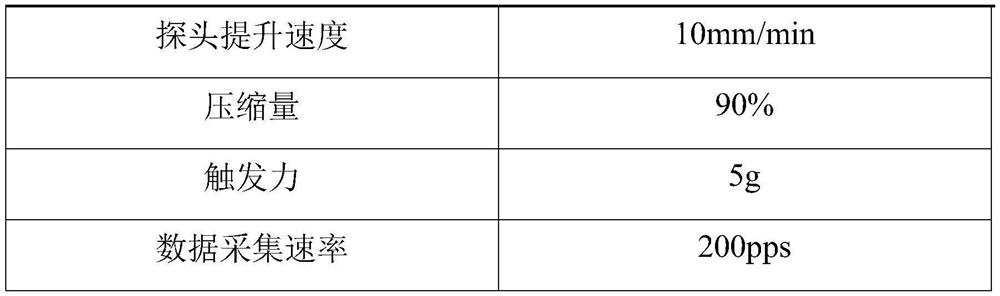
Composition with whitening effect, whitening soluble microneedle patch with high drug loading and preparation method thereof
A high drug-loading and soluble technology, applied in the field of microneedles, can solve the problems that microneedles cannot pierce the skin, low microneedle strength, and low drug-loading capacity, so as to reduce treatment costs, improve transdermal absorption rate, and improve efficacy Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The whitening soluble microneedle patch with high drug loading provided in this embodiment is composed of a needle point and a base; the raw materials of the needle point are composed as follows (in parts by weight): 13 parts of arbutin, 15 parts of magnesium ascorbyl phosphate, 7 parts of adenosine, 0.4 parts of sturgeon caviar extract, 106.2 parts of sodium hyaluronate (molecular weight of 280kDa), 6 parts of polyvinylpyrrolidone (molecular weight of 1300kDa), 12 parts of glycerin, 6 parts of hydroxypropyl cellulose; The composition of raw materials is as follows: 1.1 parts of sodium hyaluronate (molecular weight: 280Kda), 0.1 parts of glycerin, and 0.1 parts of hydroxypropyl cellulose.
[0062] The preparation method of the whitening soluble microneedle patch with high drug loading provided in this example is as follows:
[0063] S1: Preparation of needle tips: After uniformly mixing the above-mentioned raw materials for preparing needle tips, add 591.4 parts of dist...
Embodiment 2
[0066] The whitening soluble microneedle patch with high drug loading provided in this embodiment is composed of a needle point and a base; the raw materials of the needle point are composed as follows (in parts by weight): 13 parts of arbutin, 15 parts of magnesium ascorbyl phosphate, 7 parts of adenosine, 0.4 parts of sturgeon caviar extract, 70.8 parts of hyaluronic acid (molecular weight of 280kDa), 6 parts of polyvinylpyrrolidone (molecular weight of 1300kDa), 12 parts of glycerin, 6 parts of hydroxypropyl cellulose; The composition of the raw materials is as follows: 1.1 parts of sodium hyaluronate (molecular weight: 280 kDa), 0.1 parts of glycerin, and 0.1 parts of hydroxypropyl cellulose.
[0067] The preparation method of the whitening soluble microneedle patch with high drug loading provided in this example is as follows:
[0068] S1: Preparation of needle tips: After uniformly mixing the above-mentioned raw materials for preparing needle tips, add 591.4 parts of dis...
Embodiment 3
[0071] The whitening soluble microneedle patch with high drug loading provided in this embodiment is composed of a needle point and a base; the raw materials of the needle point are composed as follows (in parts by weight): 13 parts of arbutin, 15 parts of magnesium ascorbyl phosphate, 7 parts of adenosine, 0.4 parts of sturgeon caviar extract, 35.4 parts of hyaluronic acid (molecular weight: 280kDa), 6 parts of polyvinylpyrrolidone (molecular weight: 1300kDa), 12 parts of glycerin, 6 parts of hydroxypropyl cellulose; the raw materials of the base The composition is as follows: 1.1 parts of sodium hyaluronate (molecular weight: 280 kDa), 0.1 parts of glycerin, and 0.1 parts of hydroxypropyl cellulose.
[0072] The preparation method of the whitening soluble microneedle patch with high drug loading provided in this example is as follows:
[0073] S1: Preparation of needle tips: After uniformly mixing the above-mentioned raw materials for preparing needle tips, add 591.4 parts o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com