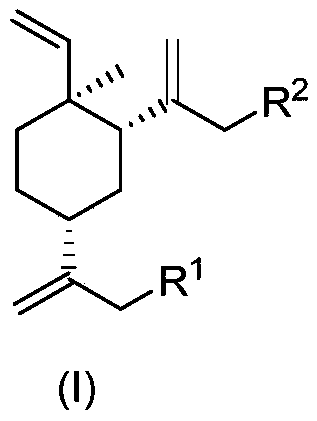
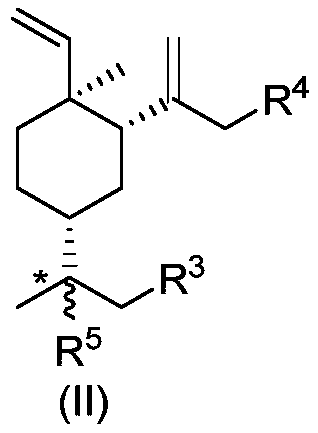
Beta-elemene halide and preparation method therefor
A technology of elemene and halogenated products, which is applied in the field of β-elemene halogenated products and its preparation, can solve the problems of complex synthesis methods, inability to separate mixtures, and difficulties in separation and purification of mixtures, and achieve high yield and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
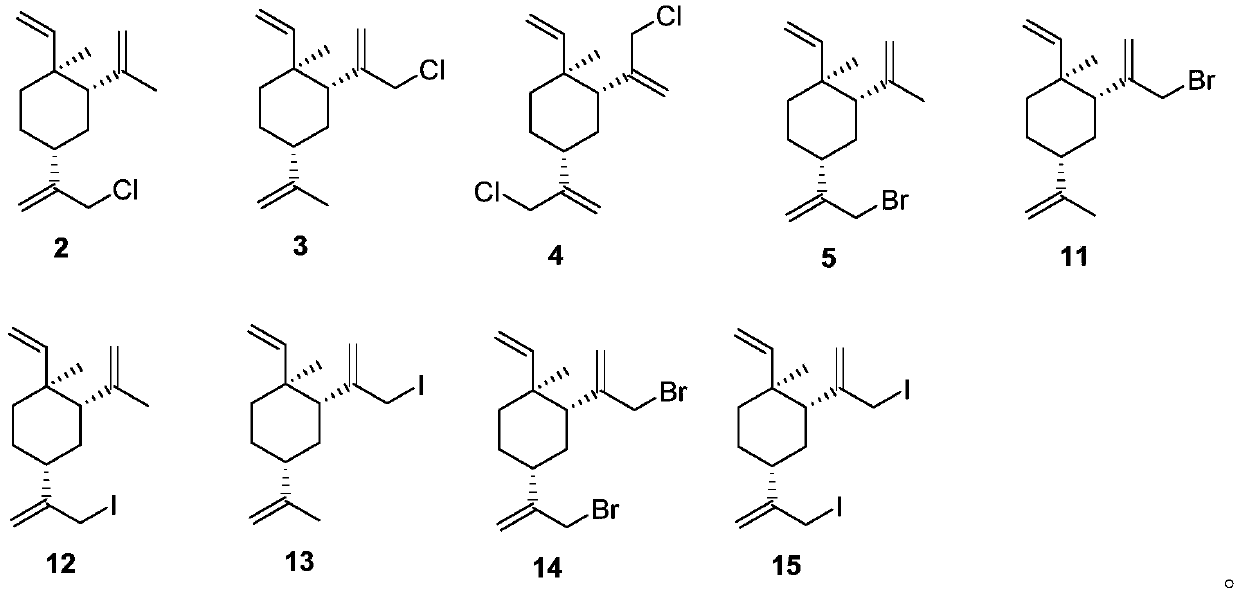
[0068] Embodiment 1: the preparation of the mixture of 13-chloro β-elemene (2) and 14-chloro β-elemene (3) (sodium hypochlorite and cerium chloride system)
[0069]
[0070] To a solution of β-elemene starting material (218mg, 1.069mmol) in dichloromethane (5mL) and water (5mL) in an ice bath, tetrabutylammonium iodide (197mg, 0.53mmol) and tributylammonium heptahydrate were added. Cerium chloride (1.153 g, 3.09 mmol). Then sodium hypochlorite (1.2 mL, content 8-13%, 3.207 mmol) was slowly added dropwise to the above mixture. The reaction solution was kept cooled in an ice bath, stirred for 0.5 hours, and reacted overnight at room temperature. The starting material was completely reacted by thin-plate chromatography, and the mixture was extracted with ethyl acetate (3 x 5 mL). The combined organic phases were washed successively with water (2 x 5 mL) and saturated brine (2 x 5 mL), and dried over anhydrous sodium sulfate. The desiccant was removed by filtration, the filt...
Embodiment 2
[0072] Embodiment 2: the preparation of the mixture of 13-chloro β-elemene (2) and 14-chloro β-elemene (3) (sodium hypochlorite and cerium chloride system)
[0073]
[0074] To a solution of β-elemene starting material (218mg, 1.069mmol) in dichloromethane (5mL) and water (5mL) in an ice bath, tetrabutylammonium iodide (197mg, 0.53mmol) and tributylammonium heptahydrate were added. Cerium chloride (1.153 g, 3.09 mmol). Then sodium hypochlorite (1.2 mL, content 8-13%, 3.207 mmol) was slowly added dropwise to the above mixture. The reaction solution was kept cooled in an ice bath, stirred for 0.5 hours, and reacted overnight at room temperature. The starting material was completely reacted by thin-plate chromatography, and the mixture was extracted with ethyl acetate (3 x 5 mL). The combined organic phases were washed successively with water (2 x 5 mL) and saturated brine (2 x 5 mL), and dried over anhydrous sodium sulfate. The desiccant was removed by filtration, the filt...
Embodiment 3
[0075] Example 3: Preparation of a mixture of 13-chloro-β-elemene (2) and 14-chloro-β-elemene (3) (NCS and cerium chloride system)
[0076]
[0077] To a solution of β-elemene starting material (201.1 mg, 1.03 mmol) in acetic acid (3 mL) was added NCS (136.7 mg, 1.00 mmol) under ice bath (10-15°C). The reaction solution was kept ice-bath cooled (10-15°C), stirred for 8 hours, thin plate chromatography detected that the raw materials were not completely reacted, and reacted overnight at room temperature (25-28°C. The reaction was slowly added dropwise with saturated sodium bisulfite (7.5mL) and Quenched with water (8mL), the mixture was extracted with petroleum ether (3 x 5mL). The combined organic phase was washed successively with saturated sodium bisulfite (3 x 5mL), water (3 x 5mL) and saturated brine (3 x 5mL) , and dried with anhydrous sodium sulfate. The desiccant was removed by filtration, the filtrate was concentrated under reduced pressure, and the resulting crude ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com