Amino acid nano hydrogel as well as preparation method and application thereof
A nano-hydrogel and amino acid technology, applied in the preparation method of peptides, chemical instruments and methods, animal/human proteins, etc., can solve the problems of multiple action sites, structural denaturation, uneven imprinted holes, etc., and achieve the preparation method The effect of simplicity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
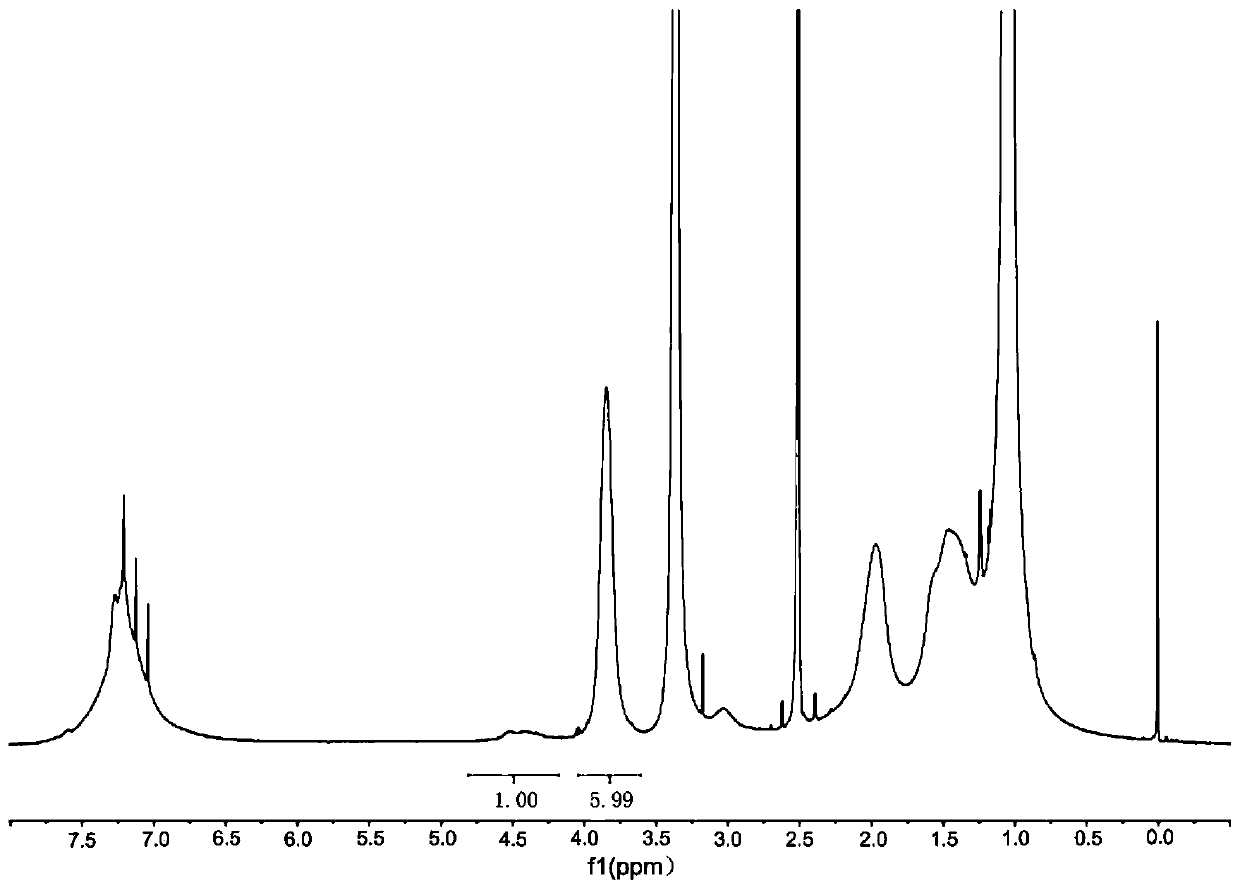
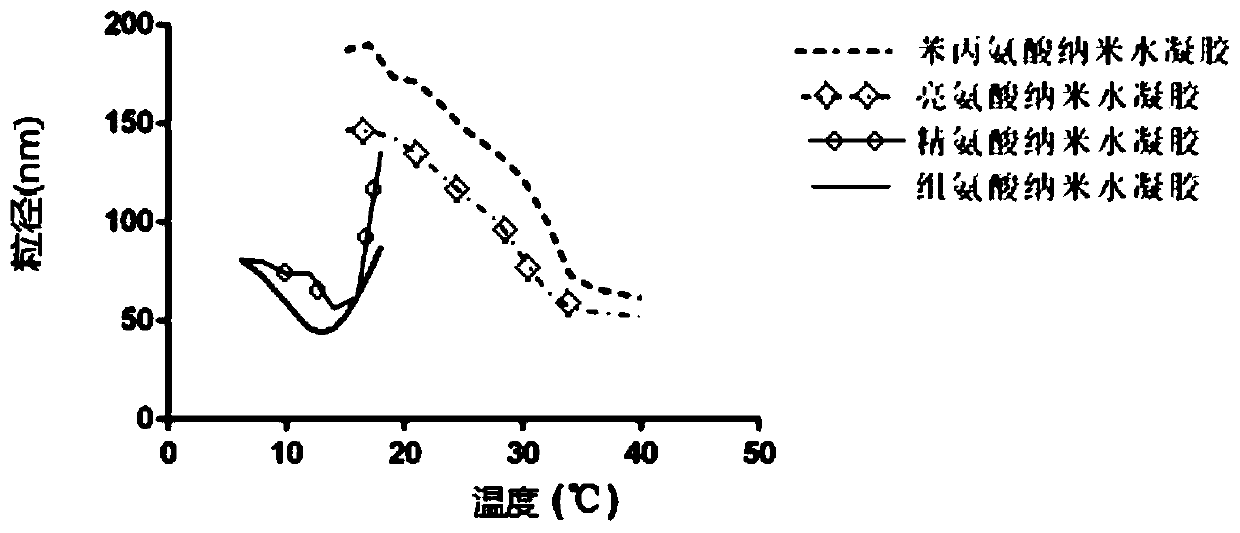
[0076] Prepare polymer A, namely hydrophobic amino acid nanohydrogel, including phenylalanine nanohydrogel and leucine nanohydrogel.
[0077] 1. Preparation of functional monomer N-acryloyl-L-phenylalanine
[0078] 3.63g (0.020mol) L-phenylalanine (L-Phe) and 1.6g (0.04mol) sodium hydroxide are dissolved in 20mL of water, under 0℃, slowly add 1.75mL (0.022mol) propylene while stirring Acid chloride, drip in 0.5h. After the addition is complete, continue stirring at room temperature for 6 hours. Adjusted to pH2 with concentrated hydrochloric acid, filtered with suction, washed with water to neutral, dried in vacuum at 50°C for 24h to obtain a white powdery solid, N-acryloyl-L-phenylalanine (APA), the yield was 45.7% (2.74) g).
[0079] 2. Preparation of functional monomer N-acryloyl-L-leucine
[0080] 2.6238g (0.02mol) L-leucine and 1.6g (0.04mol) sodium hydroxide are dissolved in 30ml of water, under 0℃, slowly add 1.625ml (0.02mol) acryloyl chloride dropwise while stirring, drop w...
Embodiment 1-2
[0088] Prepare polymer A, namely hydrophobic amino acid nanohydrogel, including phenylalanine nanohydrogel and leucine nanohydrogel. The steps for preparing phenylalanine nanohydrogel in this embodiment are as follows:
[0089] N-acryloyl-L-phenylalanine (APA) (285mg, 40mol%), N-isopropylacrylamide (213.3mg, 58mol%), N,N'-methylenebisacrylamide (10mg, 2mol%), ammonium sulfate (30mg) was dissolved in 50ml water containing 10mg sodium lauryl sulfate.
[0090] Blow nitrogen for 20 minutes, and heat in an oil bath at 65°C for 3 hours. The obtained polymer solution was put into a dialysis bag (MW 12000 ~ 14000) for 4 days and the water was changed twice a day. The dialysate inside the dialysis membrane is collected and freeze-dried to obtain phenylalanine nanohydrogel (APA@NPs).
[0091] In the same way, leucine nanohydrogels (A-Leu@NPs) were prepared, but the functional monomer N-acryloyl-L-leucine (A-Leu, 240.7mg, 40mol%) replaced N-acryloyl -L-Phenylalanine (APA).
Embodiment 2
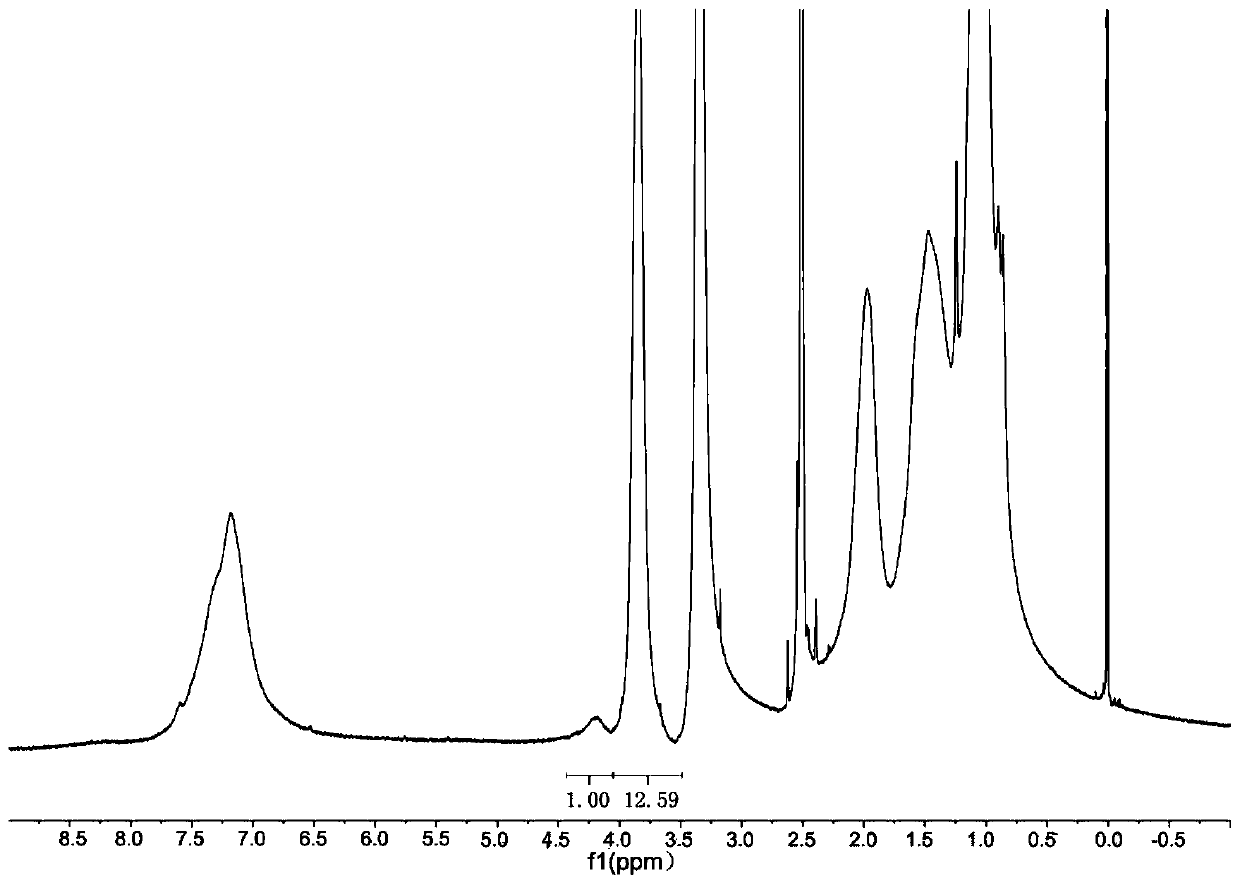
[0093] Prepare polymer B, that is, positively charged amino acid nanohydrogel, including arginine nanohydrogel and histidine nanohydrogel.
[0094] 1. Preparation of functional monomer N-acryloyl-arginine
[0095] 1.7420g (0.01mol) L-arginine and 2.12g (0.02mol) sodium carbonate are dissolved in 30ml water, placed below 0℃, slowly add 0.95ml (0.012mol) acryloyl chloride dropwise while stirring, drop within 0.5h Finish. After the addition is complete, continue stirring for 3.0 hours at room temperature. Adjust the pH to 2 with concentrated hydrochloric acid, wash with saturated sodium chloride solution, extract with ethyl acetate:isopropanol (1:1) 2×40ml, dry the organic layer over anhydrous magnesium sulfate, filter, and evaporate under reduced pressure Solvent. The residue was separated by silica gel column chromatography [V(chloroform):V(methanol)=1:1] to obtain N-acryloyl-arginine (A-Arg) as a yellow liquid with a yield of 15.00%.
[0096] 2. Preparation of functional monomer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com