Microcatheter with releasable coating at head end
A micro-catheter and coating technology, applied in the direction of catheter and coating, can solve the problems of safety risks in the catheter, and achieve the effect of excellent biological safety, avoiding potential risks and reducing the withdrawal force.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
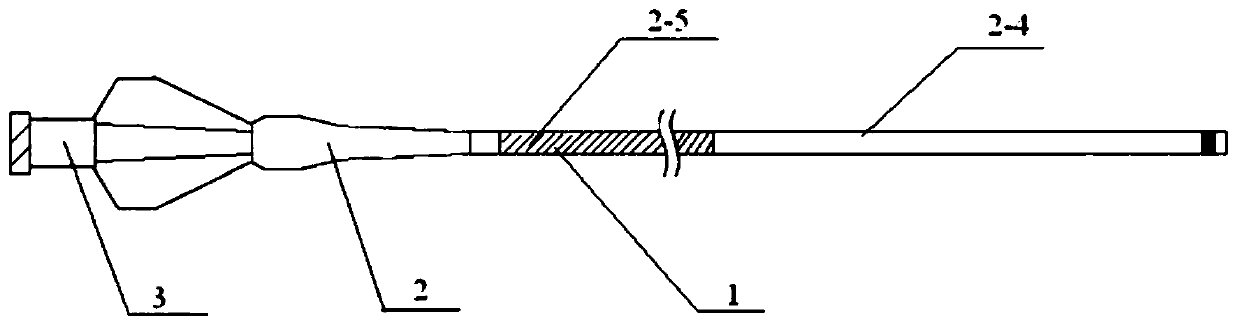
[0036] Such as Figure 1-2 As shown, this embodiment provides a microcatheter, including a microcatheter main body 1, a stress expansion tube 2 and a Luer connector 3; the microcatheter main body 1 is connected to one end of the stress expansion tube 2, and the other end of the stress expansion tube 2 It is connected with the Luer connector 3; the surface of the microcatheter main body 1 is coated with a detachable double coating.
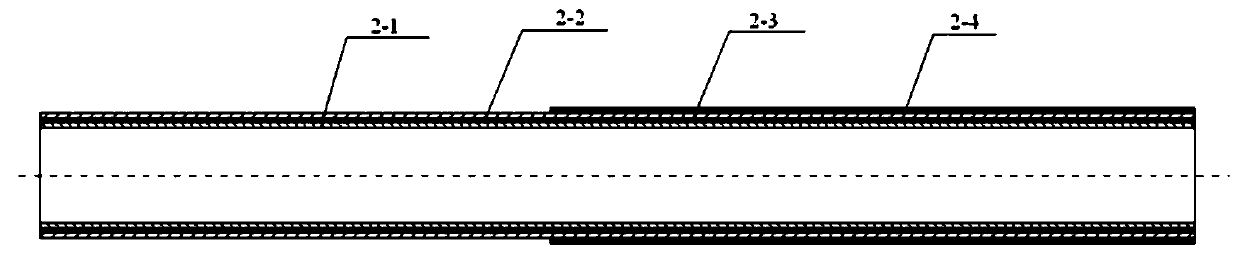
[0037] The cross-sectional structure of the microcatheter main body 1 includes an inner layer 2-1, an intermediate layer 2-2 and an outer layer 2-3 from the inside to the outside; the material of the inner layer 2-1 is a PTFE catheter, and the middle layer 2-2 It is a metal lining wire layer of braided or coiled spring structure, and the material of the outer layer 2-3 is PEBAX catheter.
[0038] The PEBAX catheter in the outer layer 2-3 is welded by several sections of PEBAX materials with different hardness; wherein the distal end material is so...
Embodiment 2
[0041] Weigh 14g of polyvinylpyrrolidone, 36g of acrylic acid, 35.5g of acrylamide, dissolve in water, add 0.01g of N,N'-methylenebisacrylamide and 0.01g of ammonium persulfate, and react at 70°C under nitrogen protection 3h, immerse the 5cm tip of the microcatheter described in Example 1 in the solution for 5s, then lift it up and away from the liquid surface at a speed of 20mm / s, place the microcatheter in an oven, and dry it at 30°C.
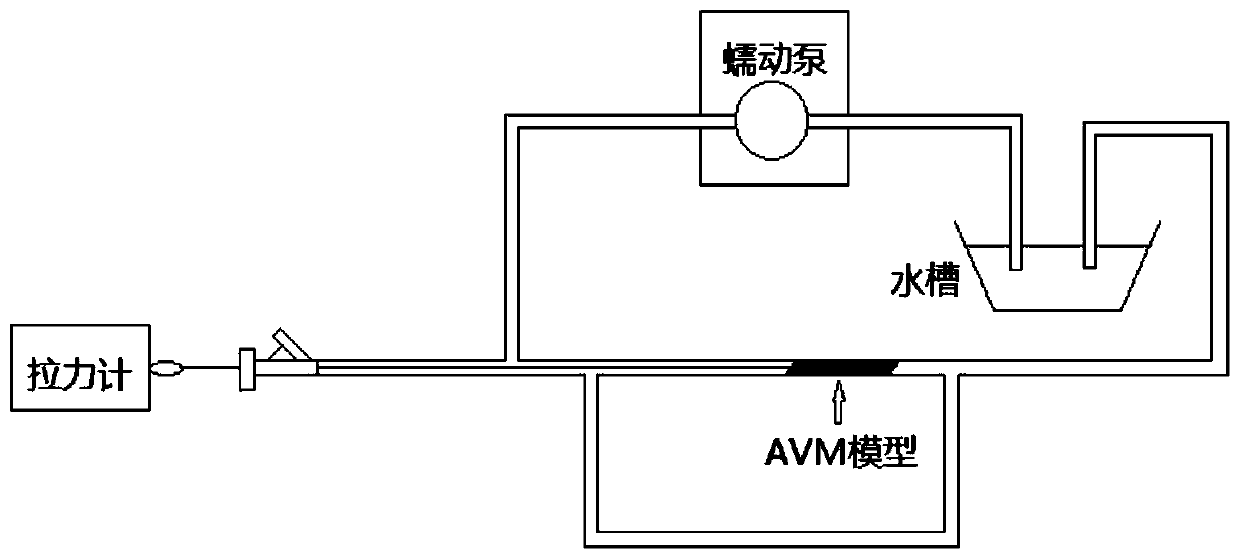
[0042] Use Onyx glue to test the withdrawal force of the microcatheter, press image 3 Build the test pipeline, the test method is as follows:
[0043] (1) Soak the dipped microcatheter in 37°C normal saline for 10 minutes;
[0044] (2) Build a microcatheter in vitro detection model as shown in the figure
[0045] (3) The water flow rate in the pipeline is 40mL / min
[0046] (4) Insert the microcatheter into the arteriovenous malformation model and make close contact with it
[0047] (5) Slowly inject the liquid embolism into the arteriove...
Embodiment 3
[0058] Weigh polyethylene glycol 10g, chitosan 10g, dissolve in deionized water containing 2% acetic acid, add a certain amount of 2 × 10-5mol / ml glutaraldehyde aqueous solution, filter and let stand for degassing, and the 1. The tip 7cm of the microcatheter is dipped in the solution for 5s, then lifted up and away from the liquid surface at a speed of 20mm / s, and the microcatheter is placed in an oven and dried at 30°C.
[0059] Using the test method described in Example 2, the measured withdrawal force of the microcatheter is 15±7g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com