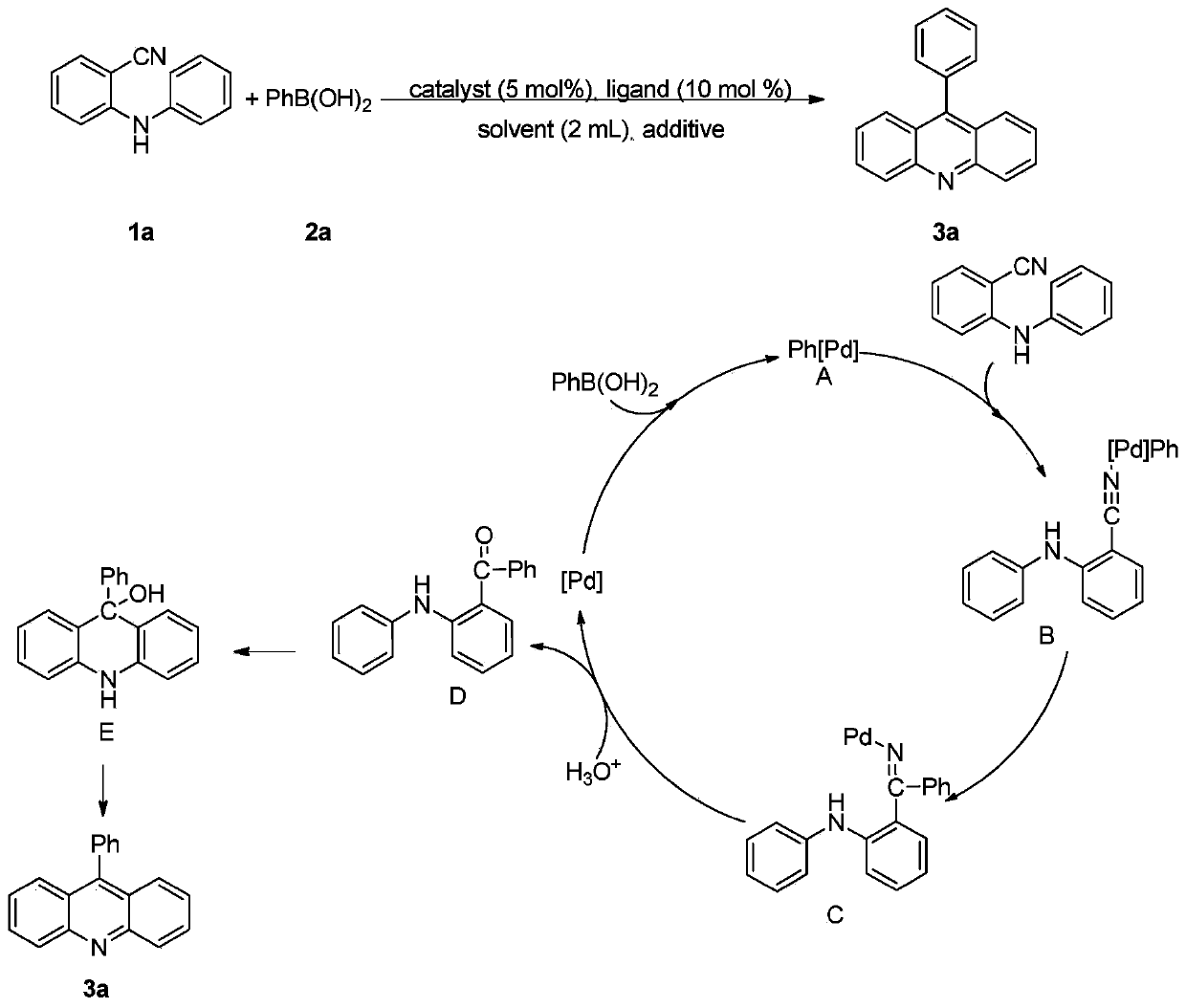
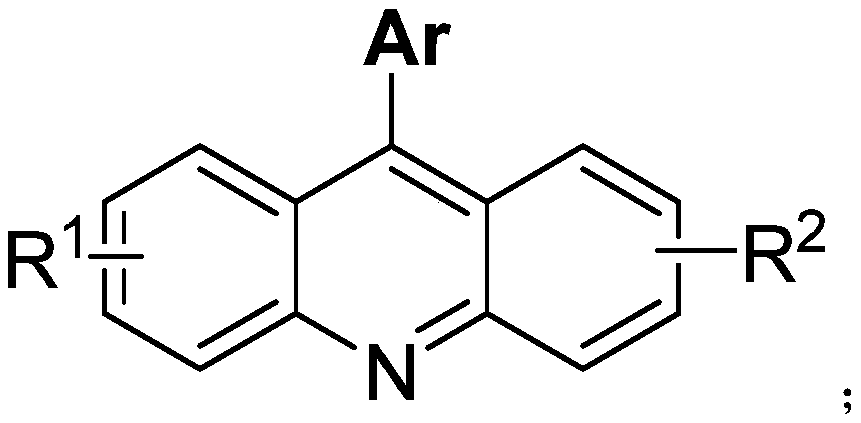
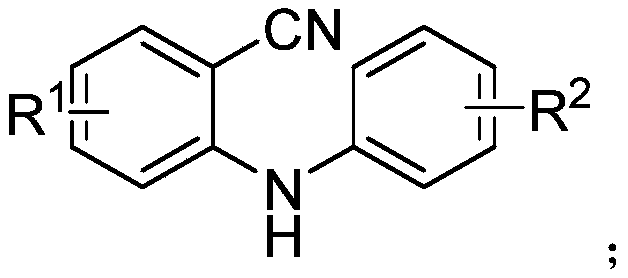
Arylacridine derivatives synthesized under palladium catalysis and preparation method thereof
A technology of aryl acridine and derivatives, applied in the field of palladium-catalyzed synthesis of aryl acridine derivatives and their preparation, can solve the problem of inability to really large-scale promotion, limitations of practicability and applicability, and inconsistent with the development of green chemistry and other problems, to achieve the effect of ensuring the health of operators, strong substrate universality, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under air atmosphere, add raw material 2-(phenylamino) benzonitrile (0.5mmol), phenylboronic acid (0.75mmol), palladium catalyst palladium acetate (5mol%), 2,2'-bipyridine ( 6mol%), methanesulfonic acid (1.0mmol) and solvent water (2mL) were stirred and mixed; after mixing uniformly, reacted at 100°C for 24h to obtain 9-phenylacridine; the product yield was 94% ;
[0032] Characterization data: 1 H NMR (400MHz, CDCl 3 )δ8.34(d, J=8.8Hz, 2H), 7.82(t, J=7.9Hz, 2H), 7.77-7.75(m, 2H), 7.76-7.63(m, 3H), 7.50-7.45(m ,4H); 13 C NMR (100MHz, CDCl 3 )δ148.8, 147.2, 136.0, 130.4, 130.0, 129.6, 128.5, 128.4, 126.9, 125.6, 125.2.9 The structural formula of phenylacridine is
[0033]
[0034] Its reaction formula is
[0035]
Embodiment 2
[0037] Under air atmosphere, add raw materials 2-(phenylamino)benzonitrile (0.5mmol), 3-chlorophenylboronic acid (0.75mmol), palladium catalyst palladium acetate (5mol%), ligand 2,2 `-Bipyridine (6mol%), additive methanesulfonic acid (1.0mmol) and solvent water (2mL) are stirred and mixed; After mixing evenly, react 24h under the condition of 100 ℃, make 9-(3-chlorobenzene base) acridine; the yield of the final product was 96%.
[0038] Characterization data:
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.30(d, J=8.8Hz, 2H), 7.81-7.77(m, 2H), 7.67(d, J=8.7Hz, 1H), 7.48-7.44(m, 3H), 7.36-7.32(m ,1H); 13 C NMR (125MHz, CDCl3) δ148.8, 145.6, 130.8, 134.0, 134.8, 130.6, 130.3, 130.0, 129.8, 128.8, 127.8, 126.6, 126.2, 125.1.
[0040] The structural formula of 9-(3-chlorophenyl)acridine is as follows:
[0041]
[0042] Its reaction formula is:
[0043]
Embodiment 3
[0045] Under air atmosphere, add raw material 2-(phenylamino)benzonitrile (0.5mmol), 4-methoxyphenylboronic acid (0.75mmol), palladium catalyst palladium acetate (5mol%), ligand 2 , 2`-bipyridine (6mol%), additive methanesulfonic acid (1.0mmol) and solvent water (2mL) are stirred and mixed; after mixing evenly, react 24h under the condition of 100 ℃, obtain 9-(4- Methoxyphenyl) acridine; the yield of the final product was 90%.
[0046] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ8.27(d, J=8.9Hz, 2H), 7.77-7.76(m, 4H), 7.13(d, J=8.3Hz, 2H), 3.94(S, 3H); 13 C NMR (125MHz, CDCl 3 )δ159.9, 149.1, 147.3, 131.9, 130.0, 129.8, 128.2, 127.1, 125.7, 125.6, 114.1, 55.6.
[0047] The structural formula of 9-(4-methoxyphenyl)acridine is as follows:
[0048]
[0049] Its reaction formula:
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com