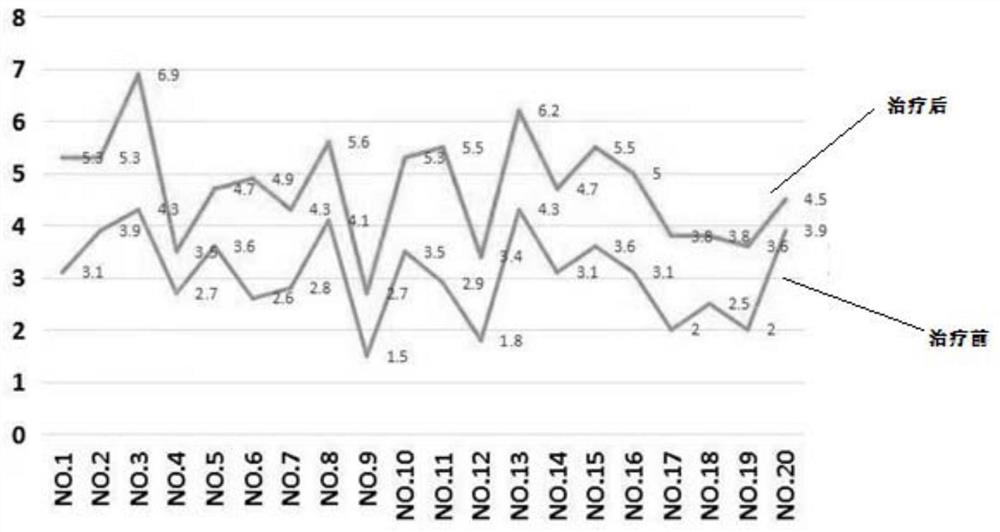
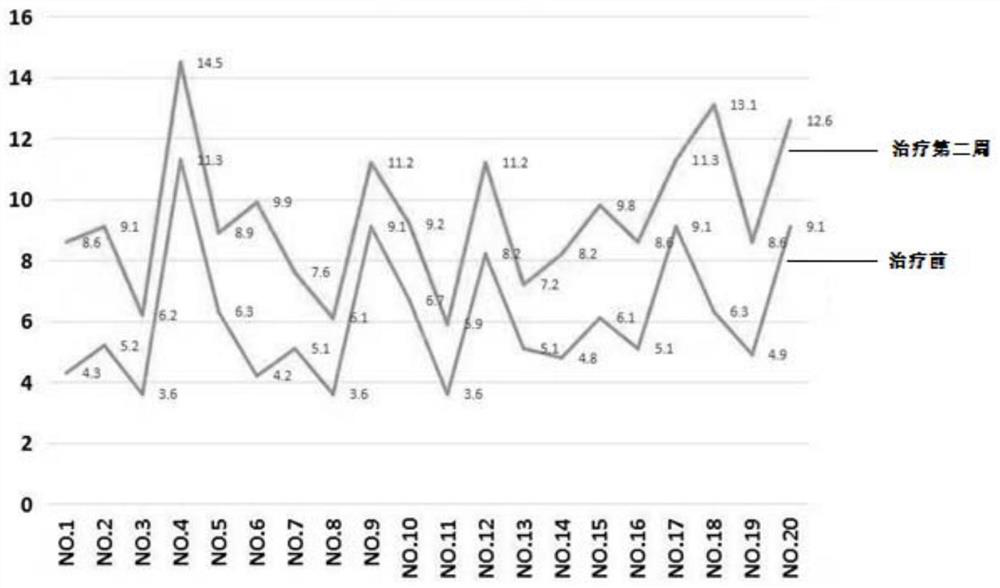
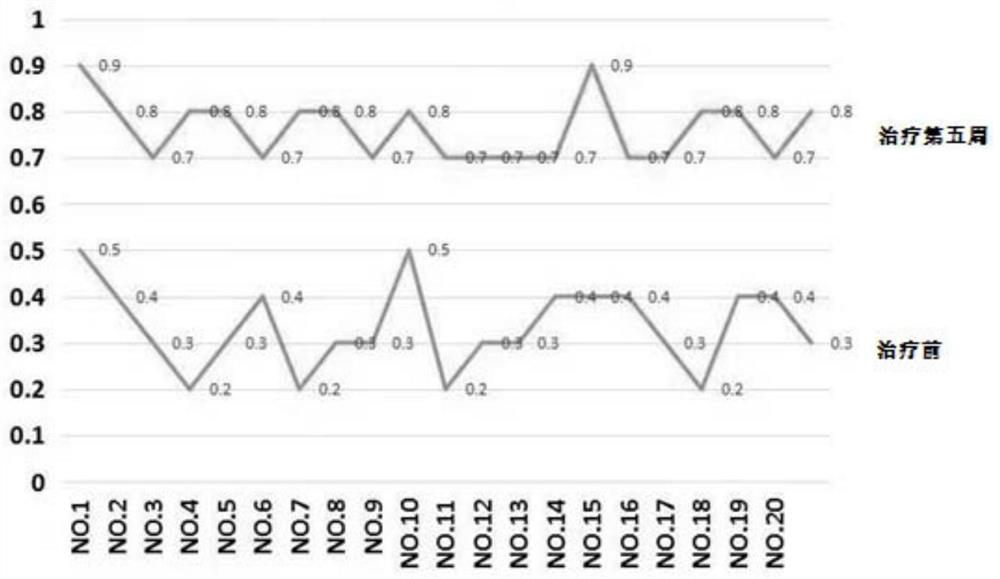
Pyrrolotriazine compounds, compositions and applications thereof
A technology of triazines and compounds, applied in the field of virus infection drugs, can solve problems such as incurable, low enzyme activity, and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Preparation of Intermediate 1
[0094] (2R)-Isopropyl 2-((perfluorophenoxy)(phenoxy)phosphoryl)alanine was prepared as follows:
[0095]
[0096] Add 10g (0.6mol) L-alanine isopropyl ester hydrochloride, 100ml dichloromethane (DCM) and 12g triethylamine (Et 3 N), cooled to -78°C, stirred for 10 minutes, and then slowly added dropwise a dichloromethane solution of phenyl dichlorophosphate (13g / 50ml). After dropping, the temperature was raised to -20°C, and the reaction was continued to stir for 1 hour. Then 13g of pentafluorophenol and 50ml of dichloromethane were added dropwise to the above reaction solution. After the dropwise addition was completed, the temperature was raised to 25°C and the reaction was stirred, and the reaction was monitored by thin-layer chromatography. After the reaction was completed, the precipitate was removed by suction filtration. The organic phase was concentrated under reduced pressure to obtain 26 g of crude product. The crude prod...
Embodiment 2
[0098] Preparation of intermediate 2
[0099] (3R,4R,5R)-2-(4-Aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-4-(benzyloxy)-5-(benzyl The preparation of oxymethyl)-3-fluorotetrahydrofuran-2-alcohol is specifically as follows:
[0100]
[0101] Add 3.4 g of 7-bromopyrrolo[2,1-f][1,2,4]-triazin-4-amine, 4.5 mL of trimethylchlorosilane (TMSCl) and 70 mL of tetrahydrofuran (THF) to the reaction flask , stirred at room temperature for 2 hours. Then the reaction solution was cooled to -78°C, 55mL, 1.0M n-butyllithium hexane solution (n-BuLi) was added dropwise, stirred for 30 minutes after the addition, and then added dropwise with 5.4g (3R, 4R, 5R)- 4-(Benzyloxy)-5-(benzyloxymethyl)-3-fluorodihydrofuran-2(3H)-one in 70 mL THF. After the dropwise addition, the reaction was stirred for 30 minutes, and then 42 mL of acetic acid was added to quench the reaction. The reaction solution was concentrated, diluted with 500 mL of ethyl acetate and washed with 250 mL of saturated NaCl solution...
Embodiment 3
[0109] Preparation of Intermediate 3
[0110] 7-((2S, 3R, 4R, 5R)-4-(benzyloxy)-5-((benzyloxy)methyl)-3-fluoro-2-methyltetrahydrofuran-2-yl)pyrrole[2 ,1-f][1,2,4]The preparation of triazin-4-amine is as follows:
[0111]
[0112] Add 3.6g of starting nucleosides and 200mL of anhydrous THF to the reaction flask, stir and cool to 0°C, add 20mL of 3N methyl magnesium chloride THF solution, and stir the reaction solution overnight. After the reaction was completed, 70 mmol of acetic acid was added to quench the reaction, and concentrated under reduced pressure to obtain a viscous liquid. The crude material was dissolved in 150 mL of anhydrous dichloromethane, 2 mL of methanesulfonic acid was added, stirred at room temperature for 12 hours, and then quenched by adding 35 mmol of triethylamine. The mixture was concentrated under reduced pressure and the residue was subjected to silica gel column chromatography to obtain 1.53 g of a methyl-substituted nucleoside.
[0113] 1 H NM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com