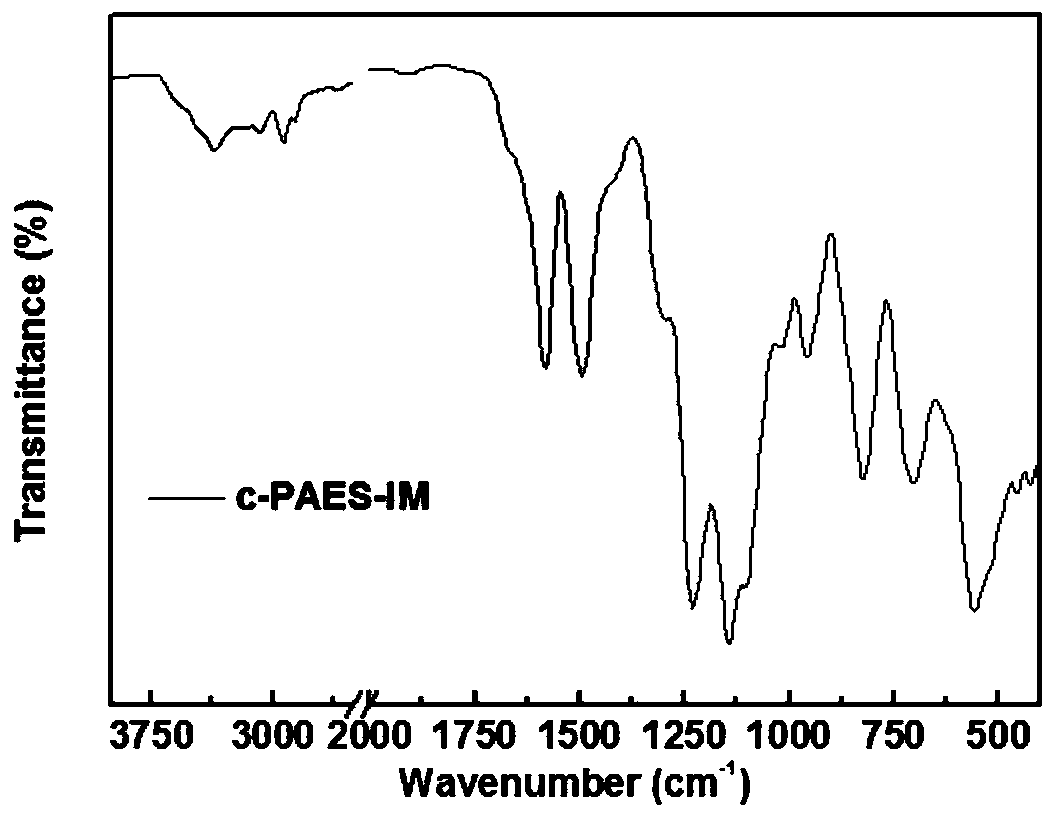
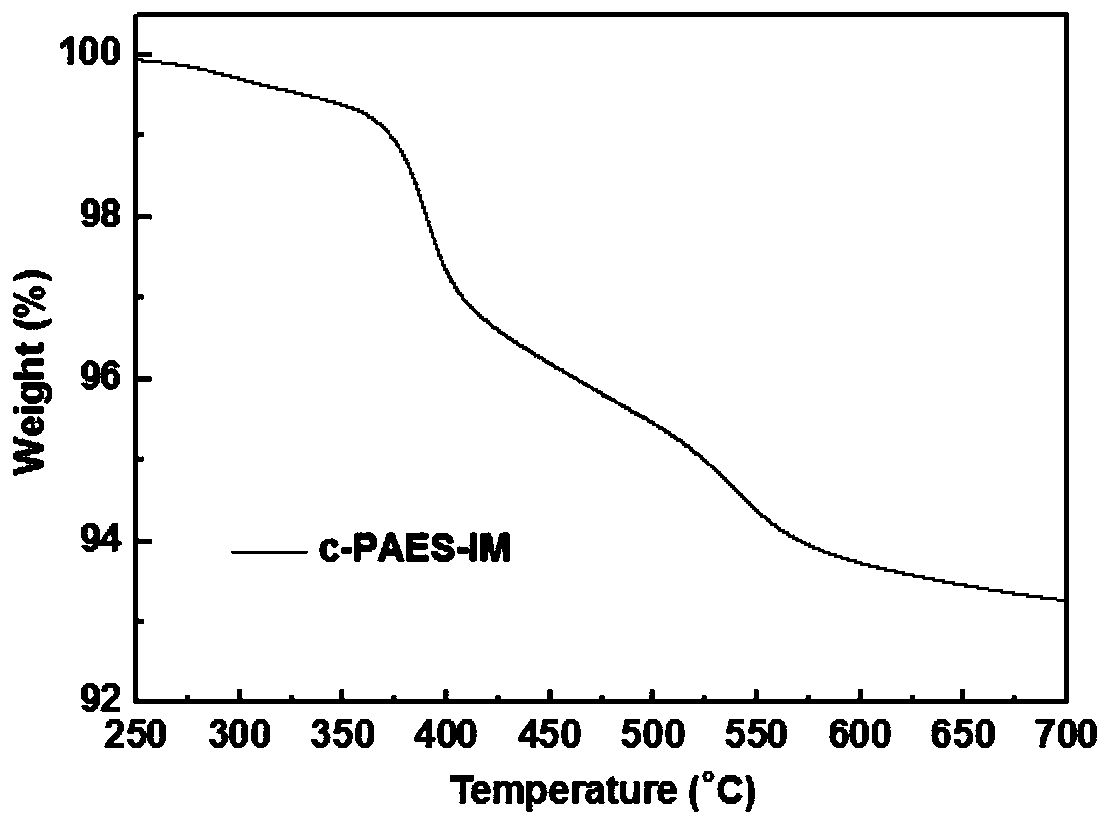
Ppreparation method for poly(aryl ether sulfone) anion exchange membrane with homogeneous cross-linked structure
A technology of anion exchange membrane and polyarylethersulfone, which is applied in the field of preparation of cross-linked structure polyarylethersulfone anion exchange membrane, can solve the problem of limited screening ability or electrostatic exclusion, performance attenuation, low permeability selectivity, etc. problems, to achieve the effect of improving chemical stability and permeation selectivity, good monovalent anion selectivity, and efficient ion transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1) Synthesis of amino-containing polyarylethersulfone: 4,4'-difluorodiphenylsulfone (20mmol, 5.0890g), 2,2'-di(4-hydroxyphenyl)hexafluoropropane (8mmol, 2.6898g ) and 2,2'-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (12mmol, 4.3951g) were added into a 250mL three-necked round bottom flask equipped with a water separator, and NMP (75mL) was used as solvent, while adding 5.5gK 2 CO 3 and 40mL toluene as catalyst and water carrier respectively. in N 2 Under the atmosphere, the reaction was carried out at 155°C for 4 hours, and further at 165°C for 3 hours. After the polymer solution dropped to room temperature, it was poured into 300 mL of isopropanol, stirred at high speed, and flocculated to obtain a precipitate. After separation by suction filtration, a brown solid was obtained, which was repeatedly washed with isopropanol and water, and dried under vacuum at 80° C. for 20 hours. The reaction yielded 8.3 g of amino-containing polyarylethersulfone with a molecular...
Embodiment 2
[0051] 1) Synthesis of amino-containing polyarylether sulfone: the same preparation process as in Example 1 was adopted to obtain amino-containing polyarylether sulfone.
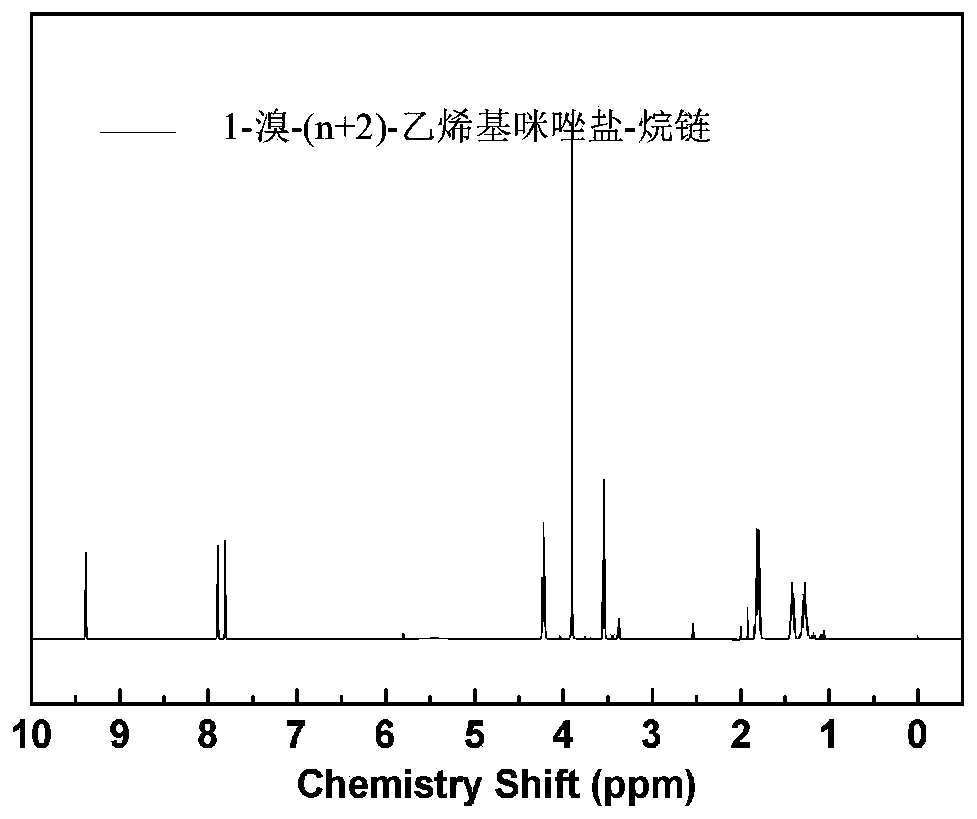
[0052] 2) Synthesis of side chains containing imidazole: In a 500mL three-necked round-bottomed flask, 1.0mmol of 1,9-dibromononane was added to 300mL of acetonitrile, heated to 40°C, and then 6.0mmol of 1- Methylimidazole, reacted for 24 hours, the resulting liquid or solid was washed several times with ether, and then vacuum-dried at 40°C for 24 hours to obtain pure 1-bromo-9-methylimidazolium salt-alkane;
[0053] In a 500mL three-neck round-bottomed flask, add 1.0mmol of 1,9-dibromononane to 300mL of acetonitrile, heat to 40°C, then add 6.0mmol of 1-vinylimidazole dropwise, and react for 24 hours to obtain The solid was washed several times with ether or ethyl acetate, and then dried under vacuum at 40°C for 24 hours to obtain pure 1-bromo-9-vinylimidazolium salt-alkane.
[0054] 3) Preparation of side ...
Embodiment 3
[0058] 1) Synthesis of amino-containing polyarylether sulfone: the same preparation process as in Example 1 was adopted to obtain amino-containing polyarylether sulfone.
[0059] 2) Synthesis of side chains containing imidazole: In a 500mL three-necked round-bottomed flask, 1.0mmol of 1,12-dibromododecane was added to 300mL of acetonitrile, heated to 40°C, and then 6.0mmol of 1 -Methylimidazole, reacted for 24 hours, the resulting liquid or solid was washed several times with ether, and then vacuum-dried at 40°C for 24 hours to obtain pure 1-bromo-12-methylimidazolium salt-alkane;
[0060] In a 500mL three-neck round bottom flask, add 1.0mmol of 1,12-dibromododecane to 300mL of acetonitrile, heat to 40°C, then add 6.0mmol of 1-vinylimidazole dropwise, and react for 24 hours. The resulting solid was washed several times with ether or ethyl acetate, and then dried under vacuum at 40°C for 24 hours to obtain pure 1-bromo-12-vinylimidazolium salt-alkane.
[0061] 3) Preparation o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com