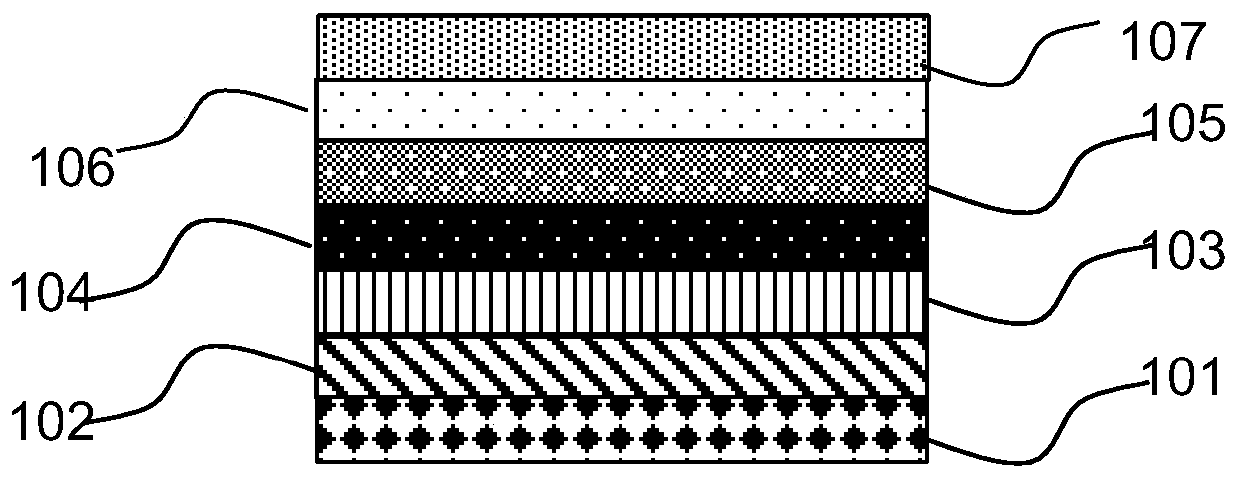
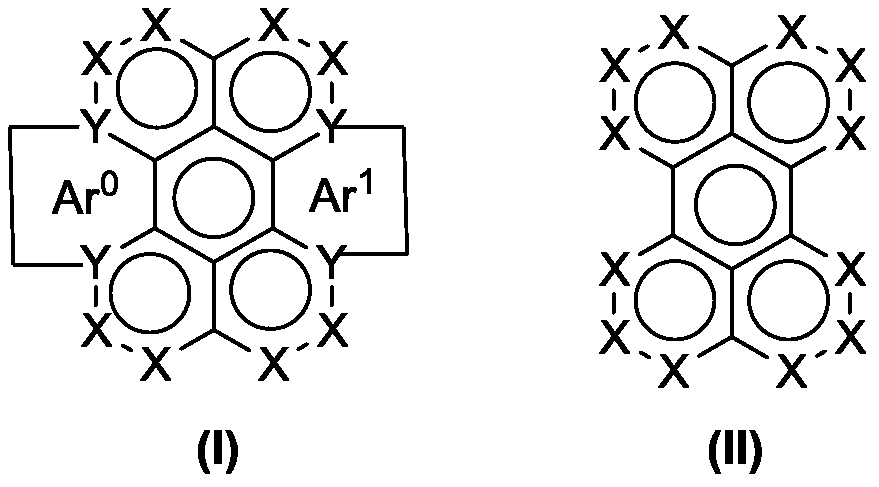
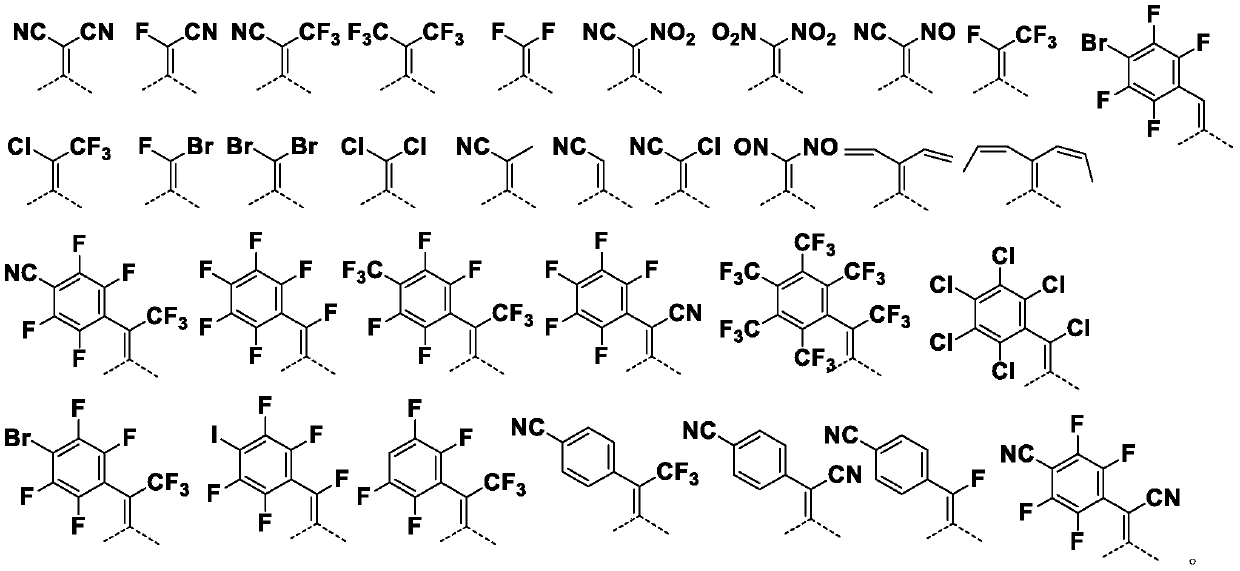
Perylene quinone-based organic compounds and application thereof
An organic compound, perylenequinone technology, applied in the field of organic electroluminescence, can solve the problems of lifespan reduction, insufficient stability, lifespan of organic light-emitting diodes, etc., to prolong device life, improve electroluminescence efficiency, and excellent hole transport Effects on properties and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] Embodiment 1 synthetic compound DPA-1
[0205]
[0206] Synthesis of compound A2:
[0207] Compound A1 (3.92g, 10mmol), 10ml of 1mol / L NaOH solution and 20ml of distilled water were stirred at 70°C for 30min, and Br 2 1.2ml continued to stir at 70°C for 24h, then removed the solvent under reduced pressure, and used dichloromethane as the eluent to pass the residue through a silica gel column to obtain the product, then removed the solvent under reduced pressure and dried the product in vacuo to prepare The desired solid compound A2 (4.85g, 67%), MS: [M+H] + =564.
[0208] Synthesis of compound A3:
[0209]Sodium tert-butoxide (2.43g, 25mmol) and malononitrile (1.32g, 20mmol) were stirred for 15min under nitrogen and dry tetrahydrofuran, and A2 (2.82g, 5mmol), Pd(PPh 3 ) 4 (3.4g, 3mmol), and cuprous iodide (3.9g, 20mmol) were then stirred at 60°C for 12 hours, then quenched with cold concentrated hydrochloric acid, concentrated in dichloromethane, dried with a...
Embodiment 2
[0212] Embodiment 2 synthetic compound DPA-2
[0213]
[0214] Synthesis of compound A4:
[0215] Sodium tert-butoxide (2.43g, 25mmol) and 4-cyano-2,3,5,6-tetrafluorobenzonitrile (4.28g, 20mmol) were stirred for 15min under nitrogen and dry tetrahydrofuran, and at room temperature Add A2 (2.82g, 5mmol), Pd (PPh 3 ) 4 (3.4g, 3mmol), and cuprous iodide (3.9g, 20mmol) were then stirred at 60°C for 12 hours, then quenched with cold concentrated hydrochloric acid, concentrated in dichloromethane, dried with anhydrous sodium sulfate, and distilled under reduced pressure , the residue was recrystallized from DCM / MeOH to afford A4 (1.28 g, 47%) MS: [M+H] + =1101.
[0216] Synthesis of compound DPA-2:
[0217] Dissolve A4 (11.00g, 10mmol) with glacial acetic acid, then cool to 0°C, then add a mixture of nitric acid and hydrobromic acid, stir at room temperature after the addition is complete, quench the reaction with distilled water, continue stirring after the solid precipit...
Embodiment 3
[0218] Embodiment 3 synthetic compound DPA-4
[0219]
[0220] Synthesis of Compound A6:
[0221] Sodium tert-butoxide (2.43g, 25mmol) and hexafluoropropane (3.04g, 20mmol) were stirred for 15min under nitrogen and dry tetrahydrofuran, and A2 (2.82g, 5mmol), Pd(PPh 3 ) 4 (3.4g, 3mmol), and cuprous iodide (3.9g, 20mmol) were then stirred at 60°C for 12 hours, then quenched with cold concentrated hydrochloric acid, concentrated in dichloromethane, dried with anhydrous sodium sulfate, and distilled under reduced pressure , the residue was recrystallized from DCM / MeOH to afford A6 (1.71 g, 81%) MS: [M+H] + =849.
[0222] Synthesis of compound DPA-4:
[0223] Dissolve A6 (8.49g, 10mmol) with glacial acetic acid, then cool to 0°C, then add a mixture of nitric acid and hydrobromic acid, stir at room temperature after the addition is complete, quench the reaction with distilled water, continue stirring after the solid precipitates, and then obtain a solid , DPA-4 (4.45g, 53%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com