Difunctional D-amino acid modified opioid peptide compound as well as synthesis method and application thereof
A synthesis method and amino acid technology, applied in the field of biomedicine, can solve problems such as loss of binding ability of compounds, and achieve the effects of enhancing analgesic ability, good pharmacological activity, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
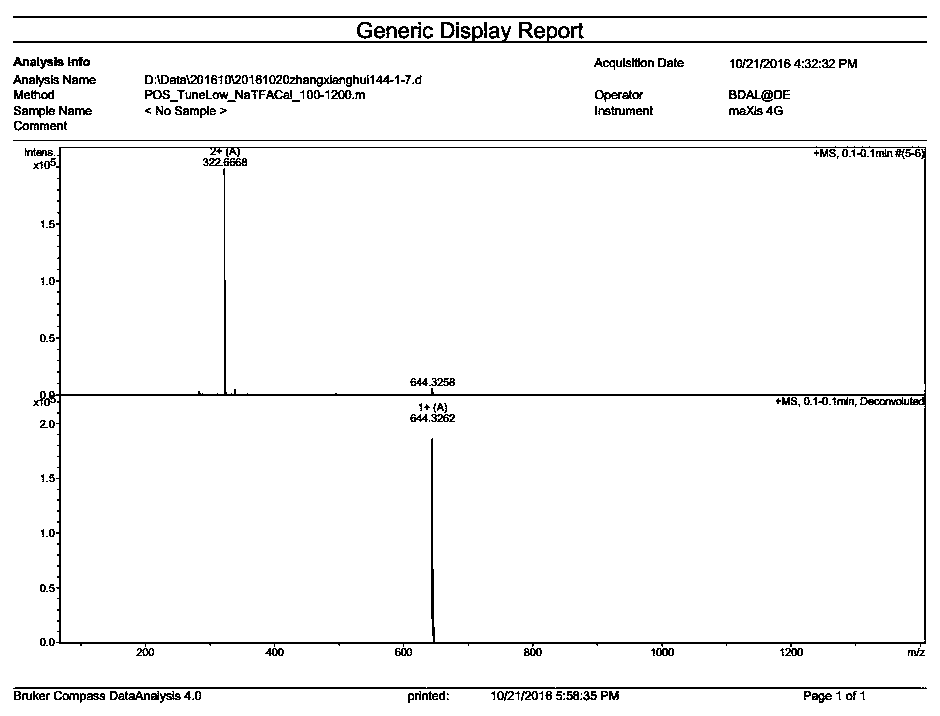
[0097] The synthesis of embodiment 1, DM24-01
[0098] (1) Resin treatment
[0099] Weigh 0.6mmol of Rink Amide-MBHA resin (substitution value is 0.44mmol / g), add dichloromethane, swell for 30min, drain, wash 3 times with N,N-dimethylformamide; add 9-fluorenylmethoxy Carbonyl removal reagent, react at room temperature for 5-15 minutes, drain, wash with N,N-dimethylformamide 3 times, and check with indene to obtain a resin from which 9-fluorenylmethoxycarbonyl protecting group has been removed.
[0100] (2) N-(9-fluorenylmethoxycarbonyl)-3-amino-2-methenyl-3-(2-furan)-propionic acid condensation
[0101] Weigh N-(9-fluorenylmethoxycarbonyl)-3-amino-2-methenyl-3-(2-furan)-propionic acid with 1.5-3 times the molar weight of the resin, and 3-6 times the molar weight of the resin 1-Hydroxybenzotriazole and O-benzotriazole-tetramethyluronium hexafluorophosphate, dissolved in N,N-dimethylformamide; subsequently, add 3-6 times the molar amount of the resin N,N-diisopropylethylamine...
Embodiment 2
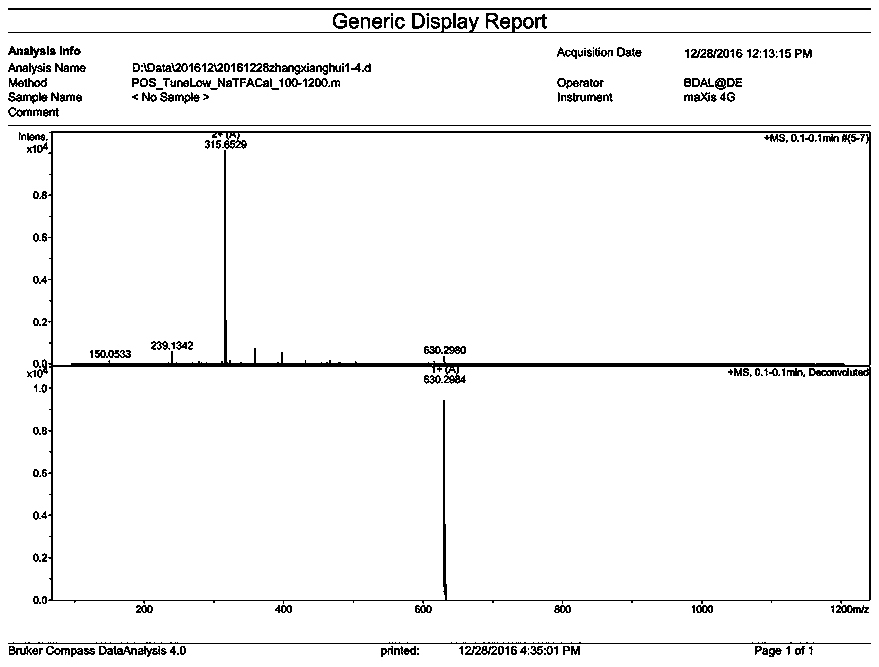
[0112] The synthesis of embodiment 2, DM24-02
[0113] (1) Resin treatment
[0114] Same as the resin treatment operation during the synthesis of compound DM24-01 in Example 1.
[0115] (2) N-(9-fluorenylmethoxycarbonyl)-3-amino-2-methenyl-3-(2-furan)-propionic acid condensation
[0116] Same as the condensation operation of N-(9-fluorenylmethoxycarbonyl)-3-amino-2-methenyl-3-(2-furan)-propionic acid during the synthesis of compound DM24-01 in Example 1.
[0117] (3)N α -Condensation of fluorenylmethoxycarbonyl-N-in-tert-butoxycarbonyl-L-tryptophan
[0118] With the N in the synthetic process of compound DM24-01 in embodiment 1 α - Condensation operation of fluorenylmethoxycarbonyl-N-in-tert-butoxycarbonyl-L-tryptophan.
[0119] (4) Condensation of N-fluorenylmethoxycarbonyl-N'-tert-butoxycarbonyl-D-ornithine
[0120] Weigh 1.5-3 times the N-fluorenylmethoxycarbonyl-N'-tert-butoxycarbonyl-D-ornithine and 3-6 times the 1-hydroxybenzotriazole of the resin molar weight, and...
Embodiment 3
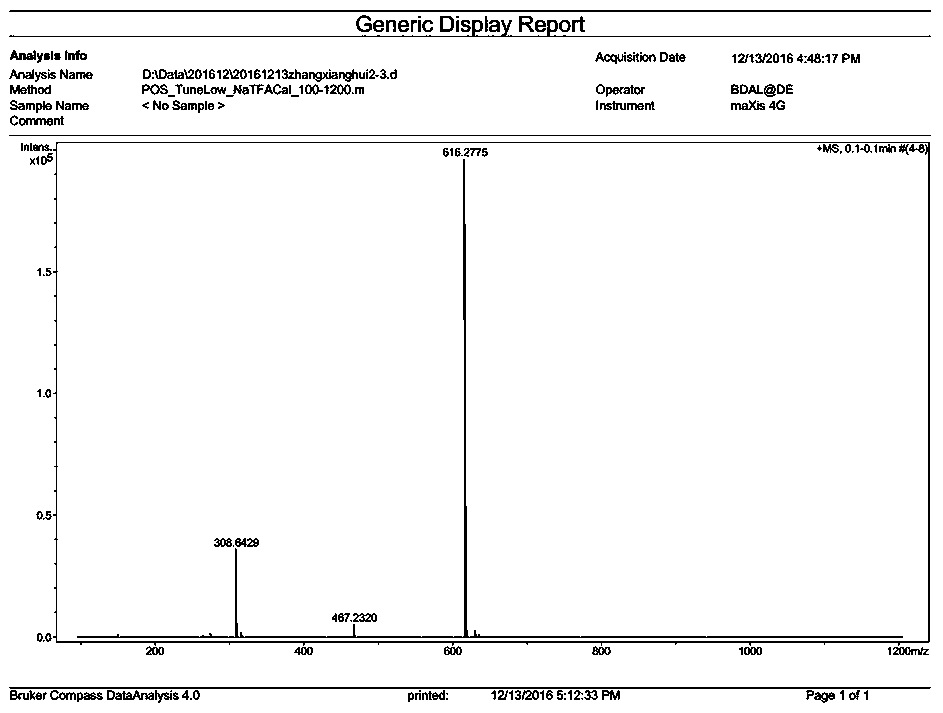
[0127] The synthesis of embodiment 3, DM24-03
[0128] (1) Resin treatment
[0129] Same as the resin treatment operation during the synthesis of compound DM24-01 in Example 1.
[0130] (2) N-(9-fluorenylmethoxycarbonyl)-3-amino-2-methenyl-3-(2-furan)-propionic acid condensation
[0131] Same as the condensation operation of N-(9-fluorenylmethoxycarbonyl)-3-amino-2-methenyl-3-(2-furan)-propionic acid during the synthesis of compound DM24-01 in Example 1.
[0132] (3)N α -Condensation of fluorenylmethoxycarbonyl-N-in-tert-butoxycarbonyl-L-tryptophan
[0133] With the N in the synthetic process of compound DM24-01 in embodiment 1 α - Condensation operation of fluorenylmethoxycarbonyl-N-in-tert-butoxycarbonyl-L-tryptophan.
[0134] (4) Condensation of (R)-2-[(9-fluorenylmethoxycarbonyl)amino]-4-[(tert-butoxycarbonyl)amino]butanoic acid
[0135] Weigh (R)-2-[(9-fluorenylmethoxycarbonyl)amino]-4-[(tert-butoxycarbonyl)amino]butanoic acid with 1.5-3 times the molar weight of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com